Chapter 50D Trauma of the Nervous System
Peripheral Nerve Trauma
Today, up to 5% of all admissions to level I trauma centers have a peripheral nerve, nerve root, or plexus injury (Noble et al., 1998). In general, injuries to the upper extremity are more common than those to the lower extremity, accounting for two-thirds of all peripheral nerve injuries. Of the four major peripheral nervous system plexuses—cervical, brachial, lumbar, and sacral—the brachial plexus is by far the most commonly affected.
Anatomy of the Spinal Nerves of the Peripheral Nervous System
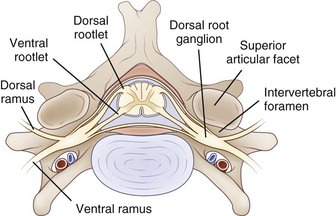
The PNS is composed of those neural elements that extend between the central nervous system (CNS—in this case the spinal cord) and their target organs (Fig. 50D.1). The peripheral motor system axons (somatic efferents) originate from the anterior horn cells and exit the spinal cord to form the ventral (anterior) rootlets. Axons of the peripheral sensory system (somatic afferent) extend from specialized sensory organs within skin, muscle, and viscera to their cell bodies, the dorsal root ganglia (DRG), which lie within the bony intervertebral foramen. These sensory fibers make up the dorsal (posterior, somatic afferent) rootlets that enter the posterior horn of the spinal cord. The mixed spinal nerves are formed when anterior and posterior rootlets combine within the neural foramen just distal to the DRG. The short spinal nerve then divides into two branches: (1) a large anterior branch (ventral ramus) that extends forward to supply the trunk muscles and gives rise to the roots of the plexus and (2) a small posterior branch (dorsal ramus) that extends backward to supply paravertebral muscles and skin of the neck.
Axon
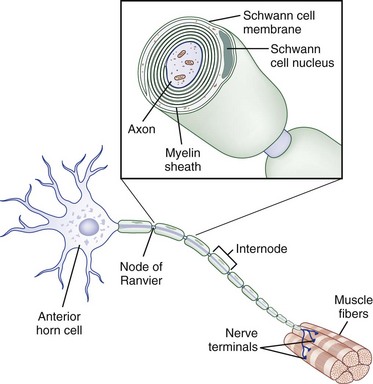
The core element of the nerve is the axon, a thin tube of axoplasm that extends from the nerve cell body to the target organ. Unmyelinated axons are partially ensheathed by invaginations of the Schwann cell membrane, whereas myelinated axons are enveloped in concentric lamellae of myelin composed of compacted spiraled Schwann cell membrane to form a sheath (Fig. 50D.2). The myelin sheath is laid down in segments called internodes, each derived from one Schwann cell. The small gap of uncovered axoplasm between sheaths is called the node of Ranvier, the site where the largest part of ion flow takes place to transmit the action potential. In saltatory conduction, action potentials leap from node to node, rather than traveling in a continuous conduction process along the entire length of the axolemma. In this way, a myelinated large-caliber axon in human adults may conduct electrical impulses at up to 73 meters per second, whereas a small unmyelinated axon may conduct as slowly as 0.5 meter per second (Kimura, 2005).
Peripheral Nerve Trunks
The connective tissue within a peripheral nerve trunk is composed of the endoneurium, perineurium, and epineurium (Fig. 50D.3). These tissues provide structure, tensile strength, and elasticity.
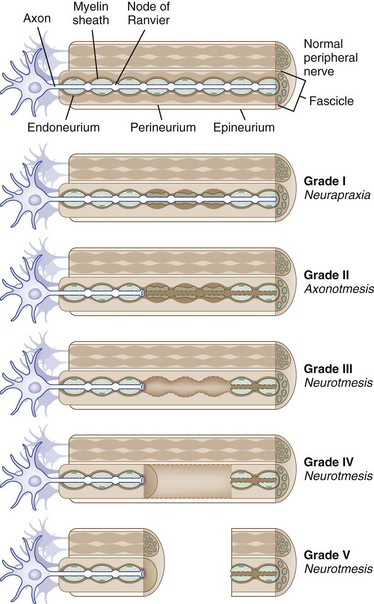
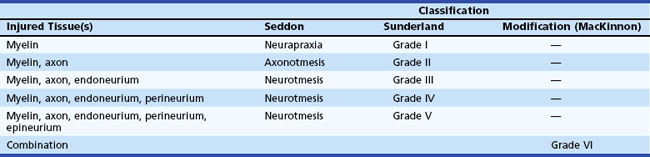
Fig. 50D.3 Classification of peripheral nerve trauma (see text).
(Image courtesy Cleveland Clinic, 2006. Illustrator, David Schumick, BS, CMI.)
Classification of Nerve Trauma
Based on observations made in Great Britain in the Second World War, Seddon devised a three-tiered classification system for nerve trauma (Table 50D.1 and Fig. 50D.3). In this system, the mildest form of injury is due to a transient focal block in conduction along the nerve fiber; injury is confined to the myelin sheath and spares the axon. He called this neurapraxia (Greek for “nonaction of nerve”). This type of injury has an excellent prognosis for complete and spontaneous recovery within a 6-week period. Indeed, many patients return to normal within hours. A clinical example of neurapraxia is wrist drop secondary to prolonged external pressure that compresses the radial nerve at the spiral groove of the humerus.
A second classification system for nerve trauma was devised by Sunderland to include additional information regarding the degree of injury to connective tissue. This system is divided into five grades: grades I and II are identical to Seddon’s neurapraxia and axonotmesis, respectively (see Table 50D.1 and Fig. 50D.3). However, Sunderland subdivided Seddon’s neurotmesis into three further levels of injury: grade III entails injury to the myelin sheath, axon, and endoneurium, with sparing of the perineurium and epineurium; grade IV describes an injury to all nerve trunk elements except the epineurium; and grade V entails complete transection of all neural and connective tissue elements of the nerve trunk. Only Sunderland’s classification system is used in the rest of this chapter.
Peripheral Nerve Degeneration and Regeneration
Large myelinated peripheral axons may respond to injury or disease in three ways: segmental demyelination, wallerian degeneration, and axonal degeneration. Segmental demyelination and wallerian degeneration are relevant to traumatic nerve injury and are discussed in more detail in this chapter, whereas axonal degeneration is more characteristically seen in metabolic and toxic nerve disorders such as diabetes mellitus and renal failure (see Chapter 76).
Wallerian Degeneration
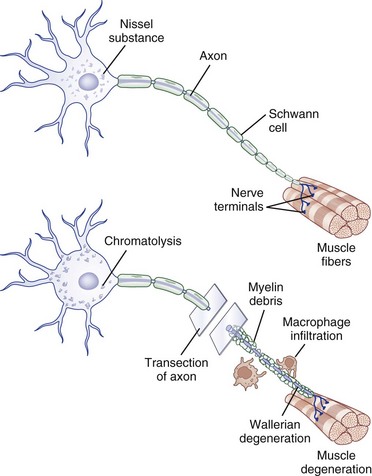
Injury to a peripheral nerve triggers a complex process that involves the axon, its cell body, its cellular connections, and the surrounding connective tissues. Wallerian degeneration follows grade II to grade V injuries and can be divided into changes that involve the segments of nerve distal and proximal to the zone of injury (Fig. 50D.4).
Distal Segment Changes
After nerve injury, the earliest changes occur in the axon distal to the site of injury, where there is disruption of retrograde and anterograde flow of signals within the axon (Makwana and Raivich, 2005). Rapid inflow of extracellular ions such as calcium and sodium occurs through the disruption in the axonal plasma membrane, which activates a cascade of events that shares features with programmed cell death or apoptosis. Axonal injury also leads to recruitment of leukocytes and initiates the cytokine-mediated signaling cascade and changes in the neighboring non-neuronal cells. This in turn triggers the synthesis of neurotrophins, chemokines, extracellular matrix molecules, proteolytic enzymes, and interleukins (Hall, 2005; Radtke and Vogt, 2009). The entire axonal process of wallerian degeneration takes approximately 1 week. By day 3, the Schwann cells retract from the node of Ranvier, and activated Schwann cells and macrophages begin to digest myelin. The process of neuronal degeneration quickly undergoes a transition to neuronal regeneration from the proximal stump. Schwann cell proliferation facilitates sprouting of new nerve branches from the injured axon terminus and initiates the sequence of events that lead to rebuilding of neural contacts to distal muscles.
Proximal Segment
Depending on the severity of injury, a limited degree of axon breakdown extends proximally from the site of injury up to the level of the first node of Ranvier. Although very proximal injury may lead to apoptosis of the cell body itself, the more common consequence of axonal injury is for the cell body to go through the process of chromatolysis. This involves the breakup and dispersion of the rough endoplasmic reticulum, the eccentric displacement of the cell nucleus, and increased nuclear expression of transcription factors that switch the pattern of gene expression from axon maintenance to protein synthesis (Dieu et al., 2005).
Nerve Regeneration
In contrast with partial or mild nerve injury, in which the surviving motor axon begins the process of collateral sprouting almost immediately, in severe or complete injury, neuronal regeneration starts from the proximal stump only after wallerian degeneration is completed (Burnett and Zager, 2004). Schwann cells play a key role in neuronal regeneration. They dedifferentiate and up-regulate the expression of adhesion molecules and neurotrophins (i.e., cadherins, immunoglobulin superfamily factors, laminin) which promote the migration of nerve sprouts that form at the regenerating axon tip. These sprouts then form cords aligned around the original basal lamina tubes of the myelinated axons (bands of Büngner) that provide a pathway along which new axons are destined to grow. The contribution of axon regeneration to clinical recovery overlaps with collateral sprouting at approximately 3 months, but axon regeneration is the main recovery mechanism from 6 to 24 months after the injury, depending in part on the distance the nerve must grow to reach its target muscle.
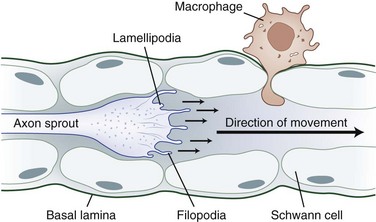
The tip of the axon sprout, called the growth cone, travels by way of filopodia and lamellipodia (Fig. 50D.5). Neurotropism, the term used to describe guidance of a regenerating axon, is accomplished by guidance molecules (i.e., semaphorins, ephrins, netrins, slits) that act to attract or repulse the growth cone to prevent misdirected growth of axon sprouts (Marx, 1995). To pass through endoneurial tubules plugged with cellular debris, the growth cone secretes plasminogen activators that dissolve cell-cell and cell-matrix adhesions. Axonal sprouts grow from the proximal to the distal stump at a rate of approximately 1 to 2 mm/day (or approximately 1 inch/month). This rate of regrowth varies depending on the location of the lesion; with proximal lesions, growth may be as fast as 2 to 3 mm/day, while that with distal lesions is about 1 mm/day.
Peripheral axons and non-neuronal cells contain growth-inducing and trophic molecules that provide the fertile milieu required for nerve regeneration. These include the neurotrophins (nerve growth factor, brain-derived neurotrophic factor, neurotrophins 3 and 4), glial cell line–derived neurotrophic factors (neurturin, artemin, persephin), insulin-like neurotrophic factor, interleukin-6, leukemia inhibitory factor, ciliary neurotrophic factor, fibroblast growth factors, and several transcription factor activators (Dieu et al., 2005; Tucker and Mearow, 2008).
Mechanisms of Traumatic Nerve injury
Compression
Mechanical compression may lead to secondary ischemic injury, as in the case of acute compartment syndromes that compromise nerve microcirculation while sparing distal-extremity pulses. When the intracompartmental pressure exceeds intraarterial pressure, nerve (and muscle) ischemia results in axon-loss lesions of variable severity. An example is the medial brachial fascial compartment syndrome after axillary arteriography or axillary regional block. Severe or irreversible single or multiple axon-loss mononeuropathies can occur within 4 hours if untreated by immediate exploration of the puncture site and decompression (Tsao and Wilbourn, 2003).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree