Traumatic Vertebral Artery Injury: Management/Workup
Robert F. Heary
Reza J. Karimi
Injury of the vertebral artery is historically uncommon; however, the routine use of advanced imaging modalities and aggressive screening has led to an increased detection of these injuries. Technical advances have also led to an increased array of therapeutic options for the treatment of vertebral artery injuries (VAIs). Despite these advances, there are no widely accepted diagnostic or treatment paradigms for this potentially devastating injury.
The anatomic course of the vertebral artery renders it susceptible to diverse mechanisms of injury. VAIs may occur as a result of penetrating neck trauma or blunt trauma, including vertebral fractures or subluxations. Iatrogenic injury of the vertebral artery may occur during surgical decompression, during placement of spinal instrumentation, or during the approach to the cervical spine. Degenerative disease, particularly of the upper cervical spine, can be associated with vertebral subluxation with resultant impingement of the vertebral artery as it courses along pathologically displaced bony prominences. Finally, neoplasms or infectious processes involving the cervical spine may result in encasement, compression, or erosion into the vertebral artery proper.
The therapeutic options are often dictated by the specific mechanism of injury. In addition, the type of vascular wall injury may also impact the choice of treatment. Unilateral vertebral artery occlusion is associated with a 12% to 24% rate of symptomatic vertebrobasilar ischemia, including stroke (1, 2, 3, 4, 5, 6 and 7). The mortality rate of vertebral artery occlusion when the patency of the contralateral vertebral artery is unknown is between 8% and 12% (1,6).
ANATOMY
The vertebral artery is anatomically divided into four distinct segments. The prevertebral segment (V1) of the vertebral artery originates intrathoracically from the superior aspect of the first segment of the subclavian artery and courses posterosuperiorly toward the bony spinal column. The artery typically enters the foramen transversarium of the sixth vertebral body and ascends vertically (V2 segment) within the transverse foramina surrounded by a fibroligamentous band, a network of autonomic nerve fibers, and a medially located venous plexus (4,8). The artery may be anchored to the exiting cervical nerve roots at the intertransverse space by arterial pedicles (9). The third segment (V3) exits superiorly from the foramen transversarium of C2 and courses posteriorly and medially along the lateral mass of C1 to enter the foramen magnum. The short intracranial segment (V4) traverses the dura of the foramen magnum and immediately enters the subarachnoid space to join the contralateral vertebral artery within the prepontine cistern. The vertebral arteries intersect to form the basilar artery ventral to the brainstem. Multiple vascular anastomoses may exist between the cervical vertebral artery and the thyrocervical trunk, and the costocervical trunk and the external carotid artery. The V1 and V3 segments are relatively mobile compared to the V2 and V4 segments, which are immobilized by bone. The most frequent anatomic anomaly of the vertebral artery (4% to 6%) involves the artery originating directly from the aortic arch. The diverse anatomy of the vertebral artery renders different anatomic segments more vulnerable to certain types of injury. The proximal V1 segment lacks bony and ligamentous protection, has an increased anterior exposure, and thus, may be more susceptible to penetrating traumatic injury than the V2 segment. The V2 segment is more frequently involved in iatrogenic injury from cervical spinal decompression or instrumentation procedures due to its intimate relationship with the intervertebral disk spaces and the posterior bony elements. The V2 segment is also rendered susceptible to direct bony impingement or arterial wall injury from displaced fractures or subluxations of the subaxial cervical spine. Arteriovenous (AV) fistulae most often occur in the V2 segment as the vessel is surrounded by a dense venous plexus during its ascent through the foramen transversarium. The V3 segment can be injured or compressed in severe degenerative or inflammatory diseases of the upper cervical spine with instability as well as due to fractures of the atlantoaxial complex and/or the occipitocervical junction due to its close apposition to these articulating bony structures. The intradural V4 segment is very rarely directly injured by blunt or penetrating trauma, but dissection of the artery in this location may occur.
PATHOPHYSIOLOGY
The sequelae of blood flow arrest through one or both vertebral arteries range from asymptomatic to posterior circulation stroke and/or death. The rate of stroke following VAI among a wide array of clinical contexts ranges from 12% to 24% (1,2). The presence of collateral circulation through the circle of Willis, the contribution of the carotid arteries to the posterior circulation blood flow, and the relative dominance of the uninjured vertebral artery all play a role in determining the neurologic outcome following a VAI. Additionally, the mechanism of injury appears to play a role in the development of symptomatic vertebrobasilar insufficiency and stroke. Ancillary factors such as patient age, the presence of other organ system injuries, and hemodynamic compromise may also effect the clinical outcome in varying degrees (1).
The paired vertebral arteries, basilar artery, posterior inferior cerebellar arteries (PICAs), anterior inferior cerebellar arteries (AICAs), superior cerebellar arteries (SCAs), posterior cerebral arteries (PCAs), and their perforating branches compose the posterior intracranial vascular circulation. The paired vertebral arteries provide an average combined net blood flow of approximately 180 mL/min as measured in healthy subjects by duplex ultrasonography. The normal variation between individuals ranges from 100 to 300 mL/min (10). Although a threshold of net flow that leads to posterior circulation ischemia has not been identified, unilateral vertebral artery flow volume of less than 30 mL/min has been interpreted as vertebral hypoplasia (10,11). Multiple extracranial sources of collateral circulation to the vertebral arteries arise from the muscular branches of the external carotid artery, the contralateral vertebral artery, and the costocervical and thyrocervical trunks. Intracranial collateral circulation is derived mainly from retrograde flow from the contralateral vertebral artery and the posterior communicating (PComm) arteries and may also arise from leptomeningeal anastomoses with the PICA, AICA, and SCA. Inadequate compensatory flow through these collateral pathways, by virtue of stenosis, hypoplasia, or injury, imparts an increased risk of stroke following vertebral artery occlusion. The PComm artery diameter is directly correlated with the risk of stroke following ligation of the vertebral and basilar arteries in the treatment of intracranial aneurysms, with the presence of two small PComms imparting the highest risk of brainstem stroke following basilar artery occlusion (3,7). In the treatment of intracranial aneurysms, therapeutic ligation of both vertebral arteries carries a rate of irreversible neurologic complications in 23%. Among those with good outcome following bilateral vertebral artery occlusion, the PComm typically supplies blood flow to the distal basilar trunk, AICA, and PICA arteries (7). In this same context, among patients who undergo unilateral vertebral artery ligation, the risk of irreversible neurologic complications is 3%. In these cases, blood flow to the posterior circulation may be supplied by the contralateral vertebral artery, in a retrograde manner, or less commonly, via the carotid circulation and PComm in cases where the remaining vertebral artery is hypoplastic.
Unilateral vertebral artery hypoplasia is observed in up to 40% of normal subjects, and the vertebral artery terminates at the PICA in up to 25% of normal subjects. In a retrospective review of 15 cases of therapeutic ligation of the vertebral artery, Hoshino reported that no patient experienced symptomatic vertebrobasilar insufficiency or stroke if the ligated artery was of equal or smaller diameter than the contralateral vertebral artery (12).
Thus, the risk of stroke following arrest of blood flow through one or both vertebral arteries is dependent on the blood flow dynamics of multiple collateral pathways. In addition, the duration of diminished blood flow can effect the development of collaterals. This may explain the higher rate of neurologic complications that occur in the setting of acute trauma. A through knowledge of these pathways and detailed investigation of the collateral circulation is critical in evaluating the patient with a VAI.
Although the mechanisms of vertebral arterial injury are diverse, vascular injury can proceed through one of several pathways resulting in flow compromise from arterial occlusion or hemorrhage with the possible development of cerebral ischemia. Vascular wall injury ranges in severity from intimal or medial damage, with or without luminal narrowing, intramural thrombus formation with luminal narrowing, dissection with or without luminal narrowing, pseudoaneurysm formation, intraluminal thrombosis, AV fistula, and complete transection. Secondary flow compromise can result from several mechanisms including progressive compression from an external hematoma, a growing pseudoaneurysm, or a free intimal dissection flap. Asymptomatic nonocclusive-type injuries, such as arterial wall dissections or pseudoaneurysms, may eventually progress to full occlusion with, or without, symptoms of vertebrobasilar ischemia. Intraluminal thrombosis, which may occur after only minor intimal disruptions, may be partial or complete and can lead to delayed embolic phenomena following their spontaneous dislodgement (13). Injuries resulting in pseudoaneurysm or AV fistulae formation may manifest with delayed hemorrhage (14). Delayed development of vertebrobasilar ischemia is not uncommon and has been reported up to several weeks after blunt traumatic and iatrogenic VAIs (1,15). This complication is thought to arise secondary to thrombus dislodgement or propagation of thrombus from an occluded vertebral artery.
TRAUMA
PENETRATING
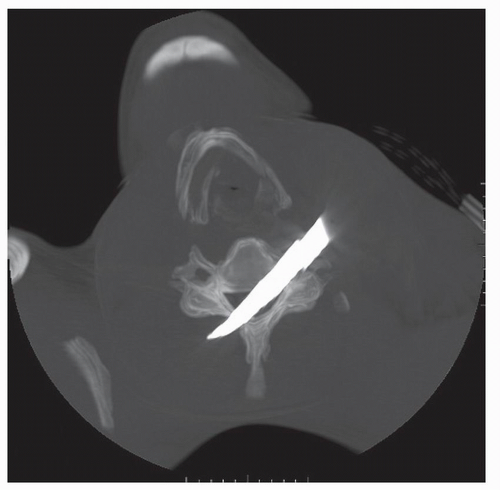
Typically, penetrating neck trauma is the result of a gunshot or stab wound and may occur in association with cervical spine fractures, ligamentous injuries, or spinal cord injuries (Figs. 52.1, 52.2 and 52.3). The incidence of VAI among patients with penetrating neck trauma ranges from 1% to 7.4% and varies according to the injury mechanism. In addition, the indications for arteriographic imaging also vary according to the specific situation (5). Concurrent injuries of the trachea, esophagus, or thoracic duct are not uncommon. Penetrating carotid artery injuries are more common than penetrating VAIs, possibly owing to its greater anterior exposure and lack
of bony protection (16). Direct penetration of the vertebral artery may result in complete arterial transection with active external hemorrhage. AV fistulae, pseudoaneurysms, arterial wall dissection, or intraluminal thrombosis may result from minor arterial wall lacerations and may proceed to delayed complete arterial occlusion and flow compromise. Concussive injury from high-velocity projectiles, without direct violation of the arterial lumen, may result in intimal or medial damage with secondary dissection or, more commonly in our experience, intraluminal thrombosis and total vessel occlusion.
of bony protection (16). Direct penetration of the vertebral artery may result in complete arterial transection with active external hemorrhage. AV fistulae, pseudoaneurysms, arterial wall dissection, or intraluminal thrombosis may result from minor arterial wall lacerations and may proceed to delayed complete arterial occlusion and flow compromise. Concussive injury from high-velocity projectiles, without direct violation of the arterial lumen, may result in intimal or medial damage with secondary dissection or, more commonly in our experience, intraluminal thrombosis and total vessel occlusion.
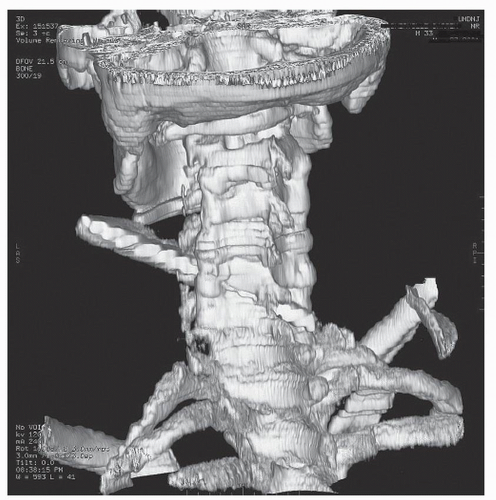 Figure 52.2. 3-D reconstructed image from the same patient in Figure 52.1. The knife blade enters and traverses the C5-C6 interspace with resultant spinal cord, vertebral artery, and ligamentous transection. |
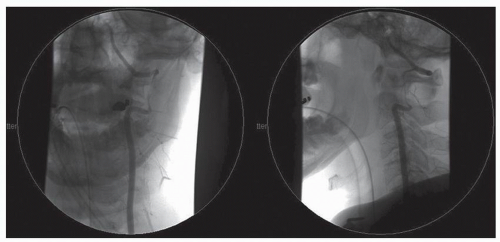
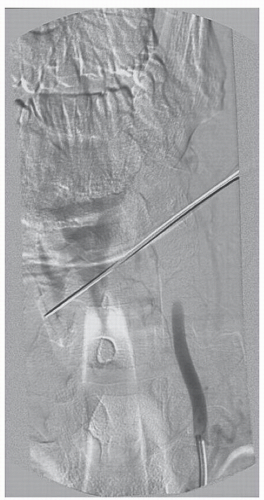 Figure 52.3. Midarterial phase anteroposterior (AP) digital subtraction angiogram of the left vertebral artery of the same patient in Figure 52.1 demonstrating complete vertebral artery thrombosis significantly inferior to the level of transection. There was no evidence of cerebral hypoperfusion or stroke. |
Penetrating neck injuries are categorized into three anatomic zones, each of which includes segments of the vertebral artery. Zone I neck injuries occur between the sternal notch and cricoid cartilage and may involve the V1 segment of the vertebral artery. Zone II neck injuries occur from the cricoid cartilage to the angle of the mandible and may involve the V2 segment. Zone III neck injuries occur between the angle of the mandible and the skull base and may involve the V3 or V4 segments of the vertebral artery.
The clinical presentation of a penetrating VAI ranges from the isolated presence of an entrance wound with or without any apparent exit wound, to “hard” signs of vascular injury such as frank external hemorrhage, hypovolemic shock, an expanding cervical hematoma, or an audible bruit. Up to 50% of patients with isolated VAI exhibit “hard signs” of vascular trauma, and 20% manifest no signs of vascular trauma (5). Neurologic deficits may or may not accompany the VAI and may result from symptomatic vertebrobasilar ischemia, concurrent spinal cord or nerve root injury, or traumatic brain injury. Importantly, multisystem trauma may mask any neurologic deficit specifically caused by vertebrobasilar insufficiency. The development of symptomatic vertebrobasilar ischemia is dictated by the degree of flow compromise of the injured vertebral artery and the presence or absence of an adequate collateral cerebral circulation.
The true incidence of vertebrobasilar ischemia in this population is not well studied but is generally regarded as a relatively rare event. One of the largest series involving 43 cases of penetrating VAIs noted that no patient presented with or developed signs of vertebrobasilar ischemia; however, there was a 4.7% mortality directly attributable to the VAI (16). In other studies, mortality rates as high as 20% have been reported (5); however, many of these cases involved major associated injuries that may have confounded these results.
TRAUMA
BLUNT
Blunt cervical trauma occurs more commonly than penetrating cervical trauma. The incidence of VAI in this population is higher than previously appreciated due to the more routine use of advanced imaging studies such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), as well as the increasing sophistication and sensitivity of these modalities. There is a relative paucity of data regarding the incidence, mechanism, treatment strategies, and outcome following blunt VAI with early reports being limited by small sample sizes. More recently, large-scale studies have yielded more powerful data on this subject.
Blunt injuries to the vertebral artery may result from spinal fractures, vertebral subluxation, facet dislocation, and direct cervical blunt force trauma (5,17,18). Mechanisms of arterial damage include vascular traction (flexion or extension distraction-type injuries), arterial impingement from displaced fracture fragments, and arterial wall violation secondary to high-grade subluxation. Traumatic dissections are due to intimal or medial damage and typically occur where the artery crosses bony prominences, such as the C1-C2 junction. Arterial dissection may also occur following chiropractic neck manipulation or other minor injuries involving sudden head movements (19) (Fig. 52.4). These types of dissection injuries are more common in the V3 segment (20). A hypothesis is that due to the V3 segment’s mobility relative to the V2 and V4 segments, which are fixed to bone, there is an increased proclivity to dissection injuries. Direct blows to the back of the neck have also been associated with isolated vertebral artery dissection, which may initially present with cervical or occipital pain (19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree