CHAPTER 391 Treatment of Other Intracranial Dural Arteriovenous Fistulas
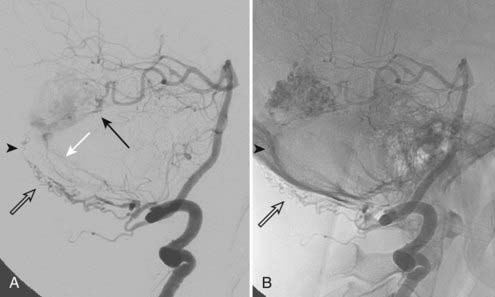
Intracranial dural arteriovenous fistulas (DAVFs) are acquired arteriovenous shunts confined to the dura and are supplied by branches of the external carotid artery, tentorial branches of the internal carotid artery, meningeal branches of the vertebral artery, and rarely, pial branches of the cerebral arteries (Fig. 391-1).1 Venous drainage is anterograde via the dural venous sinuses or retrograde into other dural or leptomeningeal venous channels. These fistulas are frequently idiopathic but can be associated with venous thrombosis,2,3 trauma, tumor, previous neurological surgery,1,4 meningitis,5 or sinus infection.1
Classification
The terms dural arteriovenous malformation and dural arteriovenous fistula are often used interchangeably. DAVFs are classified according to their venous drainage, which determines both the natural history and treatment recommendations. Houser and colleagues first established the association of clinical symptoms with patterns of venous drainage in a series of 28 patients with DAVFs.6 Although several classification systems exist for DAVFs,7–9 we refer primarily to the Borden10 and Cognard1 classifications, which have been described in detail elsewhere and are summarized in Tables 391-1 and 391-2. According to the Borden classification, type I fistulas have anterograde drainage into a dural venous sinus or meningeal vein. These fistulas are benign, often asymptomatic or characterized by a cranial bruit, and have a high rate of spontaneous remission. Borden type II fistulas have both anterograde venous sinus drainage and retrograde drainage into subarachnoid veins. The venous sinus may be patent but largely defunctionalized because of high-flow venopathy causing reversal of flow into arterialized leptomeningeal veins.
TABLE 391-1 Borden Classification of Dural Arteriovenous Fistulas
| Type I | Anterograde drainage into the dural sinus/meningeal vein |
| Type II | Anterograde drainage into the dural sinus plus retrograde drainage into the subarachnoid veins |
| Type III |
TABLE 391-2 Cognard Classification of Dural Arteriovenous Fistulas
| Type I | Anterograde drainage into the main sinus |
| Type IIa | Drainage into the main sinus with reflux into another sinus or sinuses |
| Type IIb | Drainage into the main sinus with reflux into cortical veins |
| Type IIa + IIb | Drainage into the main sinus with reflux into another sinus or sinuses plus cortical veins |
| Type III | Direct cortical venous drainage without venous ectasia |
| Type IV | Direct cortical venous drainage with venous ectasia (>5 mm and 3× larger than the diameter of the draining vein) |
| Type V | Drainage into spinal perimedullary veins |
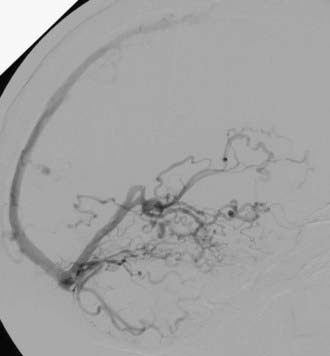
Borden type III DAVFs have exclusive retrograde drainage into arterialized subarachnoid veins at or on the wall of dural venous sinuses. Such drainage is frequently but not always due to occlusion of the arterialized dural sinus. When the sinus is patent, the point of the fistula is located either between the meningeal artery and leptomeningeal vein or between the meningeal artery and a segment of arterialized dural venous sinus that is thrombosed at either end or somehow isolated from the rest of the sinus, thereby causing reversal of drainage into leptomeningeal veins (Fig. 391-2).
Natural History
Borden type I DAVFs have a benign clinical course and a high rate of spontaneous remission. Up to 10% of Borden type II and III DAVFs with cortical venous reflux may spontaneously close as well.11 The risk for intracranial hemorrhage and stroke is dictated by the presence of cortical venous reflux, galenic drainage,11,12 and venous congestion. Lasjaunias and associates proposed that the source of intracranial hemorrhage in these cases is not the fistula itself but rather ectatic, thin-walled arterialized veins.13 Persistence of cortical venous reflux in intracranial DAVFs carries an annual risk for hemorrhagic or nonhemorrhagic neurological deficits of 15% along with an annual mortality rate of 10.4%.11 Cognard and coworkers noted intracranial hemorrhage in 40% of patients with type III and in 65% with type IV DAVFs.1
Little is known about the rebleeding rate for DAVFs, but in small case series it is as high as 43% within the first few days of hospitalization10 and up to 35% in the initial 2 weeks after the first hemorrhage.14 Davies and coauthors reported a 20% annual morbidity and mortality rate after an initial aggressive neurological manifestation.15
Treatment
Observation
Borden type I DAVFs can be observed, especially since they may spontaneously thrombose, which has been noted with cavernous sinus DAVFs.16 Factors associated with spontaneous regression of DAVFs are unknown, but sinus thrombosis may play a role.17 Occasionally, treatment is offered if a patient cannot tolerate symptoms such as a constant bruit. If conservative treatment is chosen, patients are advised to avoid antiplatelet or anticoagulant agents, if possible, to prevent interference with spontaneous thrombosis of the DAVF. High-flow venopathy in sinuses not involved in the fistulous drainage, however, may predispose to undesirable venous sinus thrombosis; such patients are advised to take at least 81 mg of aspirin daily.
Compression Therapy
Compression therapy is seldom used currently, except in patients with Borden type I fistulas as a possible first step before neuroendovascular therapy. Compression of the ipsilateral carotid or occipital artery (if the latter vessel is a known feeder to the fistula) is performed for 30 minutes at a time, three times a day. If compression of the carotid artery is chosen, patients are instructed to use the contralateral arm. In case hemispheric ischemia ipsilateral to the compressed artery and paresis of the contralateral upper extremity develop as a result of overzealous compression, the arm will fall away and the compression will necessarily be self-limited. In 25% of simple DAVFs of the transverse/sigmoid sinuses supplied by the occipital artery, compression of the occipital artery results in complete thrombosis of the DAVF within several weeks.18 In patients with subjective improvement or disappearance of pulsatile tinnitus, a follow-up cerebral angiogram can be performed to evaluate whether thrombosis of the DAVF has occurred since the last evaluation.
Transvenous Therapy
Because neuroendovascular techniques have evolved considerably over the past decade, open surgery for cure of DAVFs has become a secondary treatment. Transvenous coil embolization plus occlusion of the recipient venous pouch is the mainstay of endovascular therapy and offers the best chance of cure.19 It is particularly useful for Borden type II DAVFs, which tend to be high flow and may have multiple associated fistulas. The risk of occluding the sinus/arterialized pouch and causing venous infarction is low because the sinus is usually defunctionalized. The use of balloon test occlusion has been recommended for assessing the impact of embolizing a sinus in communication with normal cortical veins1,20,21; however, this is not our practice because all transvenous/transarterial embolizations for DAVFs at our institution are performed with the patient under general anesthesia.
As Cognard and colleagues pointed out, it is essential to differentiate between type IIb and type III DAVF (according to their classification) when considering the option of transvenous coil embolization. Transvenous treatment is appropriate for a Cognard type IIb fistula, in which there is drainage into the sinus and then reflux into a cortical vein, but it is not appropriate for a Cognard type III fistula, in which there is drainage into a cortical vein and then the sinus. In the latter case, transvenous occlusion of the sinus blocks eventual venous egress, thus further elevating pressure in the arterialized subarachnoid vein.1
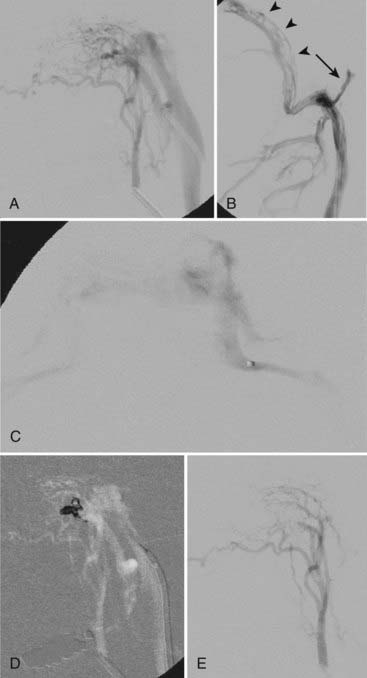
If patent, venous access is via the transfemoral ipsilateral internal jugular vein and sigmoid sinus route. A triaxial system consisting of a 6 French shuttle sheath advanced to the jugular bulb, a 4 or 5 French vertebral catheter, and a microcatheter provides maximum support. Differentiation of the recipient arterialized venous pouch from the sinus responsible for normal cortical venous drainage is crucial because in the latter drainage may occur via the sinus wall instead of the sinus.3 The arterialized sinus or fistulous pouch is then fully packed with thrombogenic fibered or platinum coils (Fig. 391-3). Inadequate embolization of a sinus with leptomeningeal venous drainage or embolization of the parent sinus without occlusion of the parallel venous channel receiving arterial inflow22 may aggravate the venous hypertension by impeding venous egress and diverting arterialized blood to the subarachnoid veins.10,19
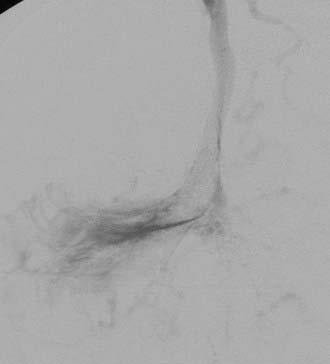
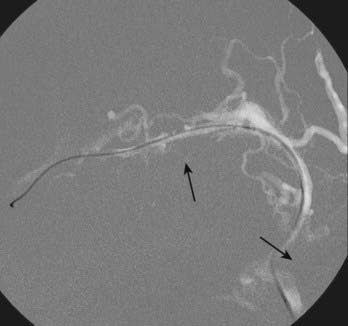
Transvenous embolization for a Borden type III fistula is successful only if the catheter can be carefully manipulated across the thrombosed or trapped segment of the sinus over a guidewire into the arterialized recipient pouch. There is a small risk of perforation and particularly subdural extravasation with this approach (Fig. 391-4).21
Sinus thrombosis is not an absolute contraindication to passage of the microcatheter,23,24 especially with the current availability of high-performance, small-caliber, hydrophilic guidewires and catheters. When entry to the diseased sinus is not feasible because of venous thrombosis, venopathy, or tortuosity, access can sometimes be secured from the contralateral transverse sinus across the torcular Herophili (Fig. 391-5).25 Recanalization of thrombosed transverse-sigmoid sinuses with balloon angioplasty plus closure of the intramural septations in the wall of the proximal sinus forming the fistula by overlapping stents has been reported. This technique may prove to be useful for eradicating DAVFs of the lateral sinus.26–28 Care should be taken to observe for alterations in venous drainage patterns after balloon angioplasty.29 Primary stent placement in a stenotic sinus to normalize venous outflow after treatment of the DAVF may also relieve venous congestive symptoms,2,30 although long-term venous stent patency rates are not yet known.
Transvenous embolization with other liquid nonadhesive agents (Eudragit E mixture) has also been described.31 In DAVFs with a dilated venous channel, creating a “nest” of coils before transvenous injection of a liquid embolic agent may prevent unwanted distal embolization.32 Proper injection technique should be used with liquid embolic agents to avoid retrograde embolization into the leptomeningeal veins, which can produce venous infarction.19
If residual opacification of the tightly packed venous pouch is noted, thrombosis can be enhanced with a 1-g/hr intravenous infusion of ε-aminocaproic acid for 24 to 48 hours, followed by a control angiogram.33 Angiographic resolution of venous hypertension correlates with the disappearance of pulsatile tinnitus and venous congestive symptoms, even if minor residual DAVF is present. However, the risk for rehemorrhage is not eliminated unless the DAVF is angiographically cured.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree