Chapter 18 Tremors
Introduction
Tremor is a rhythmic, oscillatory movement produced by alternating or synchronous contractions of antagonist muscles. It is the most common form of involuntary movement, but only a small fraction of those who shake will seek medical attention. Indeed, in one epidemiologic study of normal controls, 96% were found to have clinically detectable postural tremor, and 28% had a postural tremor of “moderate amplitude” (Louis et al., 1998e).
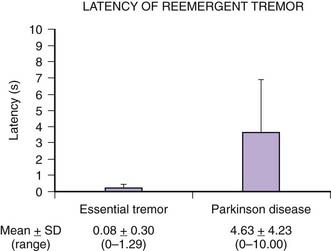
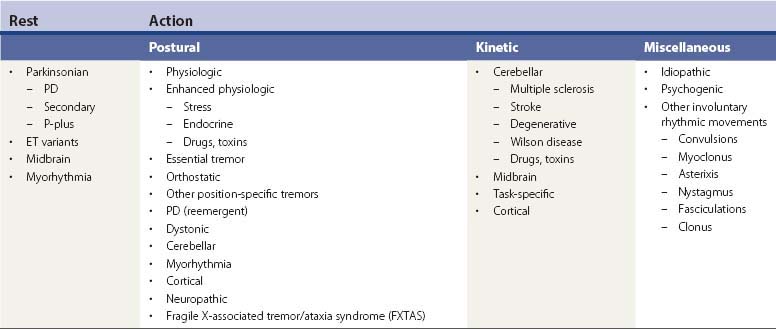
Tremors can be classified according to their phenomenology, distribution, frequency, or etiology (Hallett, 1991; Lou and Jankovic, 1991a; Bain, 1993; Findley, 1993; Deuschl et al., 1998a; Jankovic, 2000; Deuschl et al., 2001; Jankovic and Lang, 2008; Deuschl and Elble, 2009). Phenomenologically, tremors are divided into two major categories: rest tremors and action tremors (Table 18.1). Rest tremor is present when the affected body part is fully supported against gravity and not actively contracting; rest tremor is diminished or absent during voluntary muscle contraction and during movement. Action tremors occur with voluntary contraction of muscles, and they can be subdivided into postural, kinetic, task-specific or position-specific, and isometric tremors. Postural tremor is evident during maintenance of an antigravity posture, such as holding the arms in an outstretched horizontal position in front of the body. Some parkinsonian patients exhibit postural tremor that emerges after a latency of a few seconds. This tremor, referred to here as reemergent tremor, probably represents a rest tremor that has been “reset” during posture holding (Jankovic et al., 1999) (Fig. 18.1). The relationship of this reemergent tremor to the typical rest tremor is supported by the observation that this reemergent repose tremor shares many characteristics with the typical rest tremor; it has the same 3–6 Hz frequency, and it also responds to dopaminergic therapy (Video 18.1). Kinetic tremor can be seen when the voluntary movement starts (initial tremor), during the course of the movement (dynamic tremor), and as the affected body part approaches the target, such as while performing the finger-to-nose or the toe-to-finger maneuver (terminal tremor, also called intention tremor). Task-specific tremors occur only during, or are markedly exacerbated by, a certain task, such as while writing (primary handwriting tremor) (Video 18.2), while speaking or singing (voice tremor) (Rosenbaum and Jankovic, 1988; Soland et al., 1996b), or while smiling (Schwingenschuh et al., 2009). Besides writing, task-specific tremors may be triggered during other activities, such as while playing golf, particularly when putting (Video 18.3). Position-specific tremors occur while holding a certain posture (e.g., the “wing-beating” position or holding a spoon or a cup close to the mouth). One example of a task- or position-specific tremor is the tremor that occurs in performing the dot test (“dot approximation test”), during which the subject, seated at the desk with elbow elevated to a 90° shoulder abduction, is asked to hold the tip of the pen as close as possible to a dot on a horizontal paper without touching the dot. Patients with essential tremor (ET), to be discussed later, or other action tremors usually exhibit exacerbation of the tremor during this specific task. A variant of this tremor occurs in performing the modified finger–nose–finger test, during which the subject stands in front of a paper mounted on a wall and is asked to mark the center of the drawn target and to make a mark with a felt-tipped pen five times (Louis et al., 2005a). Isometric tremor occurs during a voluntary contraction of muscles that is not accompanied by a change in position of the body part, such as maintaining of a tightly squeezed fist or while standing (e.g., orthostatic tremor; see later). ![]()
Table 18.1 Classification and differential diagnosis of tremors
| A. Rest tremors |
| 1. Parkinson disease (PD) |
| 2. Other parkinsonian syndromes |
| 3. Heredodegenerative disorders |
| 4. Secondary parkinsonism |
b Drug-induced: dopamine receptor blocking drugs neuroleptics (“rabbit syndrome”), dopamine-depleting drugs (reserpine, tetrabenazine), lithium, valproate, amiodarone, flunarizine, cinnarizine f Infectious: postencephalitic, fungal, AIDS, subacute sclerosing panencephalitis, Creutzfeldt–Jakob disease |
| 5. Severe essential tremor (ET) |
| 6. Midbrain (rubral) tremor |
| 7. Tardive tremor |
| 8. Myorhythmia |
| 9. Spasmus nutans |
| B. Action tremors |
| 1. Postural tremors |
(3) Drugs: β-agonists (e.g., theophylline, terbutaline, epinephrine), dopaminergic drugs (levodopa, dopamine agonists), stimulants (amphetamines), psychiatric drugs (lithium, neuroleptics, tricyclics), methylxanthines (coffee, tea), valproate, amiodarone, cyclosporine, interferon |
| 2. Kinetic (intention, dynamic, termination) tremors |
a Cerebellar disorders (cerebellar outflow): multiple sclerosis, trauma, stroke, Wilson disease, drugs and toxins |
| 3. Task- or position-specific tremors |
| 4. Isometric |
| C. Miscellaneous tremors and other rhythmic movements |
| 1. Myoclonus: rhythmical segmental myoclonus (e.g., palatal), oscillatory myoclonus, asterixis, mini-polymyoclonus |
| 2. Dystonic tremors |
| 3. Cortical tremors |
| 4. Epilepsia partialis continua |
| 5. Nystagmus |
| 6. Clonus |
| 7. Fasciculation |
| 8. Shivering |
| 9. Shuddering attacks |
| 10. Head bobbing (third ventricular cysts) |
| 11. Aortic insufficiency with head titubation |
Tremors can be also classified according to their anatomic distribution – for example, head, tongue, voice, and trunk. Orolingual tremors include physiologic, essential, task- and position-specific, dystonic, orthostatic, parkinsonian, palatal (also termed palatal myoclonus), drug-induced, hereditary, and psychogenic (Erer and Jankovic, 2007; Silverdale et al., 2008). Because of the complexity of limb tremors, it is best to describe them according to the joint about which the oscillation is most evident – for example, metacarpal-phalangeal joints, wrist, elbow, and ankle tremor. In most tremors, the frequency ranges between 4 and 10 Hz, but the cerebellar tremors may be slower, with a frequency of 2–3 Hz. The “slow” tremors (frequency: 1–3 Hz) are sometimes referred to as myorhythmia and are usually associated with brainstem pathology (Masucci et al., 1984; Cardoso and Jankovic, 1996; Tan et al., 2007a). The “fast” tremors (frequency: 11–20 Hz) may be distinct tremor disorders, such as orthostatic tremor, or may represent harmonics of other tremors. The clinical characteristics of tremors provide the most important clues to their etiology (Table 18.1).
Assessment of tremors
There have been many attempts to quantitate tremor, but it is not apparent whether electromyographic (EMG), accelerometric, or other methods of measuring tremor correlate with clinical rating scales. Indeed, one study suggested that assessments of spirography and handwriting correlate better with overall functional tremor-related disability than electrophysiologic methods (Bain et al., 1993). However, because the physiologic measurements and the clinical ratings were not performed simultaneously and because of other technical problems, interpretation of the study is difficult. Elble and colleagues (1996) described the use of a digitizing tablet in quantification of tremor during writing and drawing. Although relatively good inter-trial correlations were obtained with this method, the tablet does not capture the speed of writing or the amount of effort exerted by the patient in an attempt to control the tremor while writing. One study found high inter-observer reliability using a diagnostic protocol for ET (Louis et al., 1998a). The investigators also found high specificity and sensitivity of a screening questionnaire when compared to the physician’s examination in patients with definite and probable ET, but actual examination of the subjects is necessary to detect mild ET (Louis et al., 1998b). In another study, the authors concluded that when a limited number of tests are available in large epidemiologic surveys, a test such as the finger–nose maneuver may be used to screen populations for ET, whereas to exclude normal subjects, the spiral drawing test, water pouring test, or arm extension test may be utilized (Louis et al., 1999a). A performance-based test for ET has been validated and compared to other measures of tremor (Louis et al., 1999b). Although this performance-based test was thought to objectively assess functional capacity in patients with ET, the test seems somewhat cumbersome to perform because it requires a variety of props, such as a milk carton, a glass, a soup spoon, a bowl, a saucer, a wallet, coins, an electrical socket, a thread and needle, a strip of buttons, and a telephone. Using other instruments, the modified Klove–Matthews Motor Steadiness Battery and the Nine-Hole Steadiness Tester, Louis and colleagues (2000c) showed that these portable instruments provide a reliable and valid means of collecting objective quantitative data on tremor severity. A Tremor Disability Questionnaire has been developed and found to reliably correlate with multiple measures of tremor severity (Louis et al., 2000a). Another screening instrument for ET, consisting of seven items and a spiral drawing, has been found to have 70.5% sensitivity, 68.2% specificity, and 64.9% positive predictive value (Lorenz et al., 2008). A simple, user-friendly clinical tool, with even higher sensitivity, specificity and predictive value, is needed to assess tremors in the clinic and in the field. A teaching videotape for assessment of ET was developed to improve the uniform application of the Washington Heights–Inwood Genetic Study of Essential Tremor (Louis et al., 2001a). Using a clinical evaluation (interview and videotaped examination) and an electrophysiologic evaluation (quantitative computerized tremor analysis using accelerometry and EMG), Louis and Pullman (2001) found a very high concordance rate between the two methods in 51 of 54 (94%) subjects, suggesting that using either technique would arrive at a similar diagnosis. Although not yet validated, the Unified Tremor Rating Assessment developed by the Tremor Research Group has been used in a number of clinical, therapeutic trials (Bain, 1993; Jankovic et al., 1996). The other scale that has been used in several tremor studies is the Fahn–Tolosa–Marin Tremor Rating Scale (TRS) (Fahn et al., 1993). While the inter-rater reliability of this scale is relatively poor, there is a good consistency, with average Spearman correlation of 0.87, when the same rater repeatedly assesses the tremor (Stacy et al., 2007). Using the TRS, Putzke et al. (2006) showed that the total score increased by about 2 points during prospective follow-up of patients with ET over a mean of 3.6 years and that older age, longer duration of disease, and asymmetric onset of tremor were associated with increased tremor severity. Another tremor rating scale, the Tremor Research Group (TRG) Essential Tremor Rating Scale (TETRAS), is currently being validated against the TRS (Elble et al., 2008). The TETRAS has been found to correlate well with quantitative assessments using the Kinesia™ (CleveMed) system (Mostile et al., 2010). Any assessment of tremor must take into account minute-to-minute and hour-to-hour amplitude variability (Koller and Royse, 1985), and potential provocations, such as voluntary isometric contraction in the case of action tremors and walking and counting backwards in the case of rest tremor (Raethjen et al., 2008).
Rest tremors
Diagnosis
Rest tremor is most typically present in patients with Parkinson disease (PD). In one study, all 34 patients with pathologically proven cases of idiopathic (Lewy body) parkinsonism demonstrated typical rest tremor sometime during the course of their illness (Rajput et al., 1991). Although this study suggests that parkinsonian patients who do not exhibit rest tremor probably do not have idiopathic parkinsonism (PD), another study, involving 100 pathologically proven cases of PD, found that 32% of all patients apparently never manifested tremor during the course of their disease (Hughes et al., 1993).
Several studies have suggested that the natural course of PD is in part related to the presence or absence of tremor (Hughes et al., 1993). The tremor-dominant PD may be associated with earlier age at onset, less cognitive decline, and slower progression than the type of PD that is dominated by postural instability and gait difficulty (PIGD) (Jankovic et al., 1990). Clinical-pathologic correlations are needed to answer the question as to whether the tremor-dominant form and the PIGD-dominant form represent different diseases or merely variants of one disease, namely, PD. In support of the former is the finding that only 27% of patients with the PIGD form of idiopathic parkinsonism had Lewy bodies at autopsy (Rajput et al., 1993). In another clinical-pathologic study, Hirsch and colleagues (1992) demonstrated that patients with PD and prominent tremor have degeneration of a subgroup of midbrain (A8) neurons, whereas this area is spared in PD patients without tremor. This observation supports the hypothesis that differential damage of subpopulations of neuronal systems is responsible for the diversity of phenotypes seen in PD and other parkinsonian disorders. It is unclear whether the occasional patients with long-standing unilateral tremor and minimal or no other parkinsonian findings have a benign form of PD, as is suggested by positron emission tomography (PET) scans showing low fluorodopa uptake in the contralateral putamen (Brooks et al., 1992), or whether this condition represents a separate disease entity. In contrast to patients with the PIGD form of PD, patients with tremor-dominant PD have increased metabolic activity in the pons, thalamus, and motor association cortices (Antonini et al., 1998). When rest tremors involve the fingers, hands, lips, jaw, and tongue in the same individual, they share a common frequency, suggesting that they are of central origin (Hunker and Abbs, 1990). This pattern, however, changes during sleep in that non-rapid eye movement sleep transforms the alternating tremor that is typically seen in the awake patient into subclinical repetitive muscle contractions of variable frequency and duration during sleep stages I to IV, and the tremor disappears during rapid eye movement sleep (Askenasy and Yahr, 1990).
Rest tremor has other causes besides PD and related parkinsonian disorders (see Table 18.1). Patients with severe ET may have tremor at rest and prominent kinetic tremor. It is not known whether the ET patients with rest tremor have associated PD, whether they later develop other features of PD, or whether the rest tremor is a feature of ET (Jankovic, 1989; Shahed and Jankovic, 2007). Some patients with lesions in the cerebellar outflow pathways, particularly in the superior cerebellar peduncle near the red nucleus (cerebellar outflow, midbrain or “rubral” tremor, also referred to as Holmes tremor), also have tremor at rest, probably due to an interruption of the nigrostriatal pathway (Remy et al., 1995). This irregular, slow (2–5 Hz), predominantly unilateral tremor may be associated with other neurologic signs, such as ataxia, bradykinesia, and ophthalmoplegia. It is often associated with midbrain pathology, such as multiple sclerosis, stroke, tumor, or arteriovenous malformation (Lee et al., 2008). It rarely responds to any medical therapy, but wrist weights, levodopa, dopamine agonists, amantadine, propranolol, clonazepam, isoniazid, and levetiracetam (Ferlazzo et al., 2008) may be effective in reducing the amplitude of this, often disabling, tremor. The affected arm or leg may be also ataxic and may be associated with third nerve palsy (Benedikt syndrome) (Video 18.4). The cerebellar outflow tremor is most often caused by trauma, stroke, multiple sclerosis, and Wilson disease (Lou and Jankovic, 1993; Krauss et al., 1995; Miwa et al., 1996; Alarcon et al., 2004). Strokes involving the posterior circulation may involve the thalamus, producing slow (1–3 Hz) rest and postural tremors, sometimes referred to as myorhythmia (Masucci et al., 1984; Cardoso and Jankovic, 1996; Miwa et al., 1996). ![]()
Myorhythmia is a slow (1–3 Hz) frequency, continuous or intermittent, relatively rhythmic movement that is present at rest but may persist during activity (Masucci et al., 1984; Cardoso and Jankovic, 1996). It may be associated with palatal myoclonus, and it disappears with sleep. Except for the slower frequency, the presence of flexion–extension rather than the typical supination–pronation pattern, and the absence of associated parkinsonian findings, myorhythmia resembles a parkinsonian tremor. In the cases that were examined at autopsy, the sites of maximum pathology involved chiefly the brainstem (particularly the substantia nigra and the inferior olive) and the cerebellum. The etiology for myorhythmia includes brainstem stroke, cerebellar degeneration, Wilson disease, and Whipple disease (Masucci et al., 1984; Tison et al., 1992; Cardoso and Jankovic, 1996).
Palatal myoclonus, sometimes referred to as palatal tremor, has some features of tremor, but in contrast to tremor which is produced by alternating or synchronous contractions of antagonist muscles, the palatal movement is produced by rhythmical contractions of agonist muscles, hence the term myoclonus is preferred despite the arguments raised against this nosology (Zadikoff et al., 2006). Palatal myoclonus, a form of segmental myoclonus, is manifested by rhythmical contractions of the soft palate resulting from acute or chronic lesions involving the Guillain–Mollaret triangle linking dentate nucleus with the red nucleus via the central tegmental tract to the inferior olivary nucleus. Symptomatic palatal myoclonus (SPM) usually persists during sleep, while essential palatal myoclonus (EPM), frequently associated with an ear-clicking sound, disappears with sleep. In EPM the muscle agonist is the tensor veli palatini, which opens the eustachian tube and is innervated by the trigeminal nerve. In SPM the palatal movement is due to contractions of the levator veli palatini, innervated by the facial nucleus and nucleus ambiguus. When the tensor muscle contracts, as in EPM, the entire soft palate moves, whereas only the edges of the soft palate move when the levator muscle contracts in SPM. Symptomatic, but not essential, palatal myoclonus is often associated with hypertrophy of the inferior olive (Goyal et al., 2000). SPM has been associated with a variety of lesions involving the brainstem as well as some neurodegenerative disorders such as Alexander disease (Pareyson et al., 2008).
Treatment with neuroleptics can also cause persistent tremor, referred to as tardive tremor (Stacy and Jankovic, 1992). This rest, postural, and kinetic tremor, with a frequency of 3–5 Hz, is aggravated by, and persists after, neuroleptic withdrawal and improves after treatment with the dopamine-depleting drug tetrabenazine. The tremor may be accompanied by other tardive movement disorders, including akathisia, chorea, dystonia, myoclonus, and stereotypy. There is usually no family history or other explanation for the tremor.
Spasmus nutans is characterized by the triad of nystagmus, abnormal head position, and irregular, multidirectional head nodding that disappears during sleep. This self-limited and often familial condition is first noted between the ages of 4 and 12 months, and it usually disappears within a year or two. Another oculomotor cause of head tremor is “head-shaking nystagmus” seen in patients with lateral medullary infarction (Choi et al., 2007). The 2–3 Hz horizontal head shaking has been postulated to be caused by unilateral impairment of nodulo-uvular inhibition of the velocity storage.
Treatment
The treatment of rest tremors is similar to that of parkinsonism (Jankovic and Marsden, 1998; also see Chapter 6). Secondary and potentially curable causes should be excluded, particularly when there are associated features to suggest disorders other than PD (see Table 18.1). Anticholinergic and dopaminergic drugs provide the most effective relief of rest tremors. Clozapine, an atypical neuroleptic that does not significantly exacerbate parkinsonism but can cause potentially serious side effects such as agranulocytosis, has been shown to be effective in the treatment of parkinsonian tremor (and ET) (Bonuccelli et al., 1997; Friedman et al., 1997; Ceravolo et al., 1999). Ethosuximide, an anticonvulsant that blocks low-threshold Ca2+ conductance in the thalamus, has been shown to reduce tremor in MPTP monkeys and to potentiate the effects of a D2 agonist (Gomez-Mancilla et al., 1992). However, ethosuximide was found ineffective in a pilot study of six PD patients with drug-resistant tremor (Pourcher et al., 1992). Mirtazapine (Remeron), a novel antidepressant that enhances noradrenergic and serotonergic transmission and acts as a presynaptic alpha-2, 5-HT2, and 5-HT3 receptor antagonist, has been reported to improve rest tremor (Pact and Giduz, 1999). Other drugs reported to have a possible beneficial effect in patients with ET include mirtazapine, clozapine, sodium oxybate, dimethoxymethyl-diphenyl-barbituric acid (T-2000), and carisbamate (Lyons and Pahwa, 2008). High-amplitude parkinsonian tremors and rest tremors caused by disorders other than PD usually do not improve with pharmacologic therapy. In some cases, botulinum toxin (BTX) injections in the involved muscles produce a satisfactory reduction in the tremor amplitude (Jankovic and Schwartz, 1991; Jankovic et al., 1996; Hou and Jankovic, 2002). A multicenter, randomized, double-blind, controlled trial confirmed the results of an earlier study (Jankovic et al., 1996) that BTX injections produce significant reduction in the postural hand tremor of ET and modest functional improvement (Brin et al., 2001). By avoiding injections of the forearm extensor muscles we prevent finger extensor weakness, a relatively frequent complication reported in the earlier studies (Pacchetti et al., 2000).
Ventral lateral thalamotomy, particularly involving the ventral intermediate nucleus of the thalamus (VIM), was considered the neurosurgical treatment of choice for disabling, drug-resistant tremors until the later 1980s when the ablative procedure was replaced by high-frequency deep brain (thalamic) stimulation (DBS) (Benabid et al., 1991; Fox et al., 1991; Jankovic et al., 1995b). Although effective in a majority of cases, the tremor recurs in about 20% of patients, and there is a considerable risk of contralateral hemiparesis, hemianesthesia, ataxia, speech disturbance, and other potential complications. These are compounded when the procedure is performed bilaterally. Thalamic DBS is now the surgical treatment of choice for patients with disabling tremors (Deiber et al., 1993; Benabid et al., 1996; Pahwa and Koller, 2001; Ondo et al., 2001a, 2001b; Pahwa et al., 2006) (see also Chapter 7). This technique has been proposed for chronic treatment of parkinsonian, essential, and other tremors. Using high-frequency (100 Hz) stimulation, with the tip of a monopolar electrode implanted stereotactically in the VIM contralateral to the disabling tremor, Benabid and colleagues (1991) noted “complete relief” of contralateral tremor in 27 of 43 (63%) thalami that were stimulated and “major improvement” in 11 (23%). The series included 26 patients with PD and 6 with ET, 7 of whom had previously been treated with thalamotomy. The benefit of thalamic stimulation was maintained for up to 29 months (mean follow-up: 13 months). The results were similar in their subsequent report of long-term effects of chronic VIM stimulation in 117 patients, 74 of whom had bilateral implantation (Benabid et al., 1996). The most robust tremor suppression was noted in patients with PD (n = 80); but patients with ET (n = 20) also benefited, although 18.5% deteriorated with time. Dysarthria and ataxia still occurred, but the patients were able to adjust the intensity of stimulation to ameliorate these side effects, though at the expense of increased tremor. Nevertheless, the investigators felt that the reversible nature of the side effects was the chief advantage of DBS over the permanent lesion produced by thalamotomy. To compare thalamic DBS with thalamotomy, Schuurman and colleagues (2000) conducted a prospective, randomized study of 68 patients with PD, 13 with ET, and 10 with multiple sclerosis. They found that the functional status improved more in the DBS group than in the thalamotomy group, and tremor was suppressed completely or almost completely in 30 of 33 (90.9%) patients in the DBS group and in 27 of 34 (79.4%) patients in the thalamotomy group. Although one patient in the DBS group died after an intracerebral hemorrhage, DBS was associated with significantly fewer complications than was thalamotomy. This procedure may be also advantageous in elderly patients and when bilateral effects are desirable (Blond et al., 1992). We found that bilateral thalamic DBS is more effective than unilateral DBS in controlling bilateral appendicular and midline tremors of ET and PD, and thalamic DBS does not seem to improve meaningfully any parkinsonian symptoms other than tremor (Ondo et al., 2001a). In addition, we found that VIM DBS produces modest improvement, rather than tremor augmentation as previously suggested, in ipsilateral tremor in patients with ET (Ondo et al., 2001b). A review of long-term efficacy of VIM DBS in 39 patients (20 with PD and 19 with ET) showed that the benefits may be maintained for at least 6 months (Rehncrona et al., 2003). In one of our patients, minimal foreign body reaction and gliosis around the electrodes was found 12 years after implantation, the longest reported follow-up with autopsy examination after DBS, supporting the long-term safety of DBS (DiLorenzo et al., 2010).
In addition to improving distal tremor associated with PD and ET, VIM DBS can effectively control ET head tremor, which usually does not respond to conventional therapy (Koller et al., 1999). Other midline tremors, such as voice, tongue, and face tremor, also may improve with unilateral VIM DBS, although additional benefit can be achieved with contralateral surgery (Obwegeser et al., 2000). The risk of local gliosis with chronic stimulation of the thalamus is minimal (Caparros-Lefebvre et al., 1994). Unfortunately, thalamic stimulation does not appear to be as effective in patients with predominantly kinetic and axial tremors, and it does not improve other parkinsonian features such as bradykinesia, rigidity, and levodopa-related motor complications. Furthermore, while VIM DBS is very effective in improving PD tremor, when performed bilaterally it is often associated with dysarthria and postural and gait abnormality (Pahwa et al., 2006) as a result of which the subthalamic nucleus (STN) has been suggested as a more appropriate target in PD patients with severe tremor (Limousin et al., 1998; Benabid et al., 2000; Diamond et al., 2007; Fishman, 2008). The mechanism of action of DBS is unknown, but “jamming” of low-frequency oscillatory inputs has been suggested as a possible mechanism for the antitremor effects of DBS. Regional cerebral blood flow, measured by PET scan, demonstrated that tremor suppression was associated with decreased cerebellar blood flow and, presumably, decreased synaptic activity in the cerebellum (Deiber et al., 1993).
In 1992, Laitinen of Stockholm, Sweden, reported the results of 90 pallidotomies in 86 patients with severe PD (Laitinen et al., 1992). The external, posteroventral portion of the medial globus pallidus interna (GPi) was the intended target for the stereotactically placed lesion. Nearly all patients had “marked improvement in tremor and akinesia.” In addition, some patients apparently also noted improvement in their gait, speech, and pain. Only two patients suffered permanent visual field defect, and one had “minor stroke with hemiparesis.” Several pallidotomy series have since confirmed the beneficial effects of pallidotomy on various parkinsonian symptoms, including tremor (Jankovic and Marsden, 1998). These results provide support for the notion that the GPi is “hyperactive” in PD and that surgical or chemical lesions of these structures may have a therapeutic value not only in controlling tremor but also in improving bradykinesia (Bergman et al., 1990; Aziz et al., 1991). Although some investigators (Subramanian et al., 1995) have suggested that posteroventral pallidotomy is as effective as thalamotomy in controlling parkinsonian tremor, others (Dogali et al., 1995) feel that pallidotomy provides only partial relief of tremor.
Postural tremors
Diagnosis and clinical features
Physiologic tremor
Normal and enhanced physiologic tremors are the most common forms of postural tremor, but they rarely require medical attention. Postural tremors are clinically similar despite different etiologies. In contrast to ET, the frequency of physiologic tremor can be slowed by mass loading (Elble and Koller, 1990). Indeed, there appear to be two components to physiologic tremor: variable frequency (peak: 8 Hz), which is dependent on loading, and consistent frequency (peak: 10 Hz), which is independent of peripheral influence. The latter suggests central origin of the tremor, as is presumed the case in ET. Thus the amplitude of ET is less dependent on the position of the tested limb than is the amplitude of other postural tremors, including physiologic tremors (Sanes and Hallett, 1990).
Essential tremor
Although the term “essential” implies necessary or desirable, it actually means that there is no known cause and the term is synonymous with “idiopathic.” The term “essential tremor” did not gain regular and widespread currency until a century or so after its initial use in 1874 by Pietro Burresi, a professor of medicine at the University of Siena, Italy (Louis et al., 2008b). He coined the term “tremore semplice essenziale” or “simple essential tremor” when he described the case of an 18-year-old man suffering from severe tremor of the arms when engaged in voluntary movement as well as head tremor. While the amplitude of ET tends to increase with age, the tremor frequency decreases with age (Elble et al.,1994; Elble, 2000b). The tremor of ET is typically a postural or kinetic tremor with frequency varying between 4 and 10 Hz. Although the frequency of the tremor is relatively constant in a particular individual, the amplitude may vary and in some cases may be even suppressed by mental concentration and distraction (Koller and Biary, 1989; Kenney et al., 2007).
Epidemiology of ET
In the past the modifier “benign” was used (“benign ET”) to indicate favorable prognosis of ET, even though it is now well accepted that ET can produce marked physical and psychosocial disability (Busenbark et al., 1991; Jankovic, 2000; Sullivan et al., 2004; Louis, 2005; Benito-León and Louis, 2006; Elble et al., 2006). Furthermore, in a longitudinal, prospective, population-based study, ET has been found to be associated with increased mortality at an estimated risk ratio of 1.59 (95% CI 1.11–2.27, P = 0.01) (Louis et al., 2007a). Meta-analysis of epidemiologic studies has found the prevalence of ET to range between 0.01% and 20.5%, but the pooled prevalence is 0.9%; the prevalence in people ≥65 years old is 4.6% and may be as high as 21.7% in people ≥95 years old; the prevalence is higher in males than females (Louis and Ferreira, 2010). There are many other estimates (Haerer et al., 1982; Louis et al., 1995, 1998d; Dogu et al., 2003) such as 5.5% in people over the age of 40 years (Rautakorpi et al., 1982) and 14% in people 65 years old or older (Moghal et al., 1994) (Table 18.2). In one epidemiologic study, 108 of 1056 (10%) nondemented individuals in upper Manhattan, aged 65 years or older, reported “shaking” (Louis et al., 1996). Neurologic examination confirmed rest tremor in 8.3% and action tremor in 17.6%, and the prevalence of PD and ET was estimated to be 3.2% and 10.2%, respectively. In a door-to-door survey of people aged 40 years or older in Mersin Province, Turkey, the prevalence of ET was found to be 4% (Dogu et al., 2003). In another population-based survey, involving 5278 subjects aged 65 years or older in central Spain who were followed for a median of 3.3 years, the adjusted annual incidence was determined to be 616 per 100 000 person-years; 64 of the 83 (77.1%) incident cases had not been previously diagnosed, and only 4 (4.8%) were taking antitremor medications (Benito-León et al., 2005). In yet another population-based study of northern Italian adults in which all participants were examined and classified by movement specialists using rigorous diagnostic criteria, tremors comprised the most common category of movement disorders, followed by restless legs syndrome (Wenning et al., 2005). These epidemiologic studies provide strong evidence that the prevalence and incidence of ET are higher than was previously recognized.
Table 18.2 Prevalence and incidence of essential tremor
| Prevalence |
| Incidence |
Diagnosis of ET
Although ET was described as early as the nineteenth century (Dana, 1887; Louis, 2010), there is still considerable controversy about the diagnostic criteria for ET (Chouinard et al., 1997; Louis et al., 1998c; Jankovic, 2000; Louis, 2010; Quinn et al., 2011). In one study of 71 patients, 37% diagnosed with ET based on the criteria for ET adapted from the consensus statement of the Movement Disorders Society (Deuschl et al., 1998a) were misdiagnosed, usually either as PD or dystonia (Jain et al., 2006). This is partly due to a lack of a disease-specific marker for ET. No specific pathologic changes indicative of PD were noted in 20 brains of ET patients that were examined at autopsy (Rajput et al., 2004). However, this study is fundamentally flawed, since all patients who were selected for the study had a diagnosis of ET at the time of death and patients who started with ET and later developed PD would have been excluded (Jankovic, 2004). It is of interest that one patient with severe ET that began at age 45 and no parkinsonian features, at the time of her death at age 91 years had Lewy bodies localized to the locus coeruleus, providing further evidence of a connection between ET and Lewy body disease (Louis et al., 2005b). The controversy about the possible association of ET and PD should be clarified once the genetic basis and pathophysiology of ET are understood. Until then, the operational diagnostic criteria must rely on the presence of typical clinical characteristics. The presence or absence of certain clinical characteristics may be used to categorize ET into “definite,” “probable,” and “possible” (Table 18.3). The diagnostic criteria may be used or modified according to specific needs. For example, for genetic linkage studies, only “definite” ET may be acceptable, whereas in studies that are designed to explore the clinical spectrum of ET, including associated features, the “possible” ET category might be more appropriate (Table 18.4). Family history, alcohol sensitivity, and propranolol responsiveness, while characteristic of ET, should not be considered necessary for the diagnosis. More recently, core and secondary criteria were proposed to facilitate a practical approach to the diagnosis of ET (Elble, 2000a). Core criteria include bilateral action tremor of the hands and forearms (but not rest tremor), absence of other neurologic signs, except for the Froment sign (a “cogwheel” phenomenon on passive movement of the affected limb with voluntary movement of the contralateral limb), and isolated head tremor without signs of dystonia, although the latter is rare. A recent analysis of ET patients from two large population-based studies and one large clinic-based cohort revealed no cases of pure head tremor in 583 patients (Louis and Dogu 2009). Therefore, patients with pure head tremor probably should not be regarded as definite ET. Head tremor is seen in about 18% of population-based cases of ET and in 37% of clinical samples of ET (Louis and Dogu, 2009). Secondary criteria include long duration (>3 years), a positive family history, and a beneficial response to alcohol (Mostile and Jankovic, 2010). Another feature, seen in about a third of patients with ET, is mirror movements (Louis et al., 2009d), more typically observed in patients with focal dystonia (Sitburana et al., 2009). There are red flags that indicate a diagnosis other than ET, such as unilateral tremor, present in only 4.4% of cases of ET (Phibbs et al., 2009), leg tremor, rigidity, bradykinesia, rest tremor, gait disturbance, focal tremor, isolated head tremor with abnormal posture (head tilt or turning), sudden or rapid onset, and drug treatment that may cause or exacerbate tremor. Thus head tremor is usually a manifestation of ET or cervical dystonia; it is almost never seen in PD unless there is coexistent ET (Roze et al., 2006; Gan et al., 2009).
Table 18.3 Proposed classification of essential tremor
| A. Definite essential tremor |
| 1. Inclusions |
a Bilateral postural tremor with or without kinetic tremor involving hands or forearms, which is visible and persistent and is long-standing in duration (>5 years) |
| 2. Exclusions |
| B. Probable essential tremor |
| 1. Inclusions |
| 2. Exclusions |
a Primary orthostatic tremor, which is an isolated, high-frequency (14–18 Hz), bilaterally synchronous leg tremor on standing or voluntary contraction of leg muscles |
| C. Possible essential tremor |
| 1. Inclusions |
a Type 1. Satisfy criteria for definite or probable ET but exhibit other recognizable neurologic disorders, such as: |
| 2. Exclusions |
Members of the Tremor Research and Investigation Group: M. Brin, C. Contant, R. Elble, L. Findley, J. Jankovic, W. Koller, P. LeWitt, A. Rajput. From Findley LJ, Koller WC. Definitions and behavioural classifications. In Findley LJ, Koller WC (eds): Handbook of Tremor Disorders. New York, Marcel Dekker, 1995, pp 1–5.
Table 18.4 NIH Essential Tremor Consortium diagnostic criteria for essential tremor
Tremor rating: 0, none perceived; 1, slight (barely noticeable); 2, moderate, noticeable, probably not disabling (<2 cm excursions); 3, marked, probably partially disabling (2–4 cm excursions); 4, severe, coarse, disabling (>4 cm excursions).
Participants in the July 1996 NIH meeting: J. Beach, S.B. Bressman, M.F. Brin, D. De Leon, L. Goldfarb, M. Hallett, J. Jankovic, W. Koller, D. Mirel, K. Wilhemsen. From Brin MF, Koller W. Epidemiology and genetics of essential tremor. Mov Disord 1998;13(Suppl. 3),55–63.
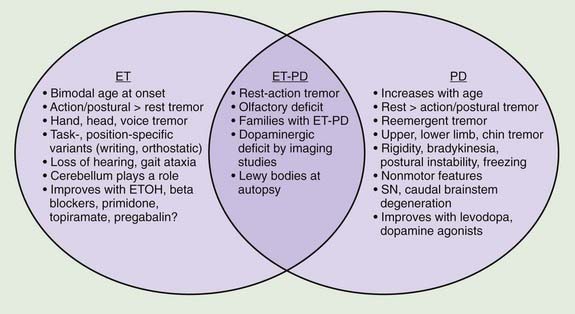
A review of the clinical features in 350 consecutive patients who were referred to the Movement Disorders Clinic at Baylor College of Medicine and diagnosed with ET has shown that although tremor is clearly the most troublesome symptom, it is not necessarily the only symptom in patients with ET (Lou and Jankovic, 1991b) (Tables 18.1 and 18.3). This is supported by the reports of well-studied families in which some members have typical ET, while others have dystonia, parkinsonism, or a combination of all three disorders (Jankovic et al., 1997; Farrer et al., 1999; Bertoli-Avella et al., 2003; Yahr et al., 2003; Spanaki and Plaitakis, 2009). One multigenerational family, 36 members in five generations, had an admixture of ET, PD, and dystonia (Yahr et al., 2003). Two twin brothers with ET and PD had the classic pathologic features of PD at autopsy. The authors concluded, “This unusual set of clinical and pathologic circumstances can hardly be attributed to chance occurrence and raises the question of a specific genetic mutation and/or clustering, which may link ET with PD.” In a study of the first-degree relatives of 303 PD probands and 249 controls from Crete, ET was present in the relatives of PD patients more often than in those of controls (OR: 3.64, P < 0.001) and the risk was even greater (OR: 4.48) when the affected proband had tremor-dominant or mixed PD (Spanaki and Plaitakis, 2009). Twelve subjects had both ET and PD phenotypes. The authors concluded that “in certain families ET and PD are genetically related probably sharing common hereditary predisposition.” Retrospective chart review at the Neurological Institute (NI) of New York showed that 56.7% of 210 PD patients versus 33.3% of 210 Parkinson-plus syndrome patients (P < 0.001) had kinetic tremor on examination and patients with PD were more likely to have a diagnosis of ET assigned by an NI neurologist (5.3% vs. 0.0%, OR 12.85, 95% CI 1.66–99.8, P = 0.001) (Louis and Frucht, 2007). Patients with PD were three to thirteen times more likely to have diagnoses of ET than patients with Parkinson-plus syndromes, thus confirming “the link between ET and PD, and possibly, between ET and Lewy body disease.” In another large family, originally from Cuba, manifested by parkinsonism and ET, the parkinsonism was linked to a marker on chromosome 19p13.3–q12, but it did not cosegregate with ET (Bertoli-Avella et al., 2003). Other gene mutations associated with PD have not been found in patients with ET alone (Deng et al., 2006a). Some, but not all (Adler et al., 2011) studies have suggested that there is an association between ET and dystonia and between ET and parkinsonism (Jankovic et al., 1997; Yahr et al., 2003; Shahed and Jankovic, 2007; Fekete and Jankovic, 2011) (Fig. 18.2). In addition to dystonic tremor, patients with dystonia frequently have postural ET-like tremor present in body parts distal to the dystonia, and they have a higher-than-expected family history of postural tremor (Chan et al., 1991; Jankovic et al., 1991; Deuschl et al., 1997; Jankovic and Mejia, 2005; Schneider et al., 2007). Asymmetric dystonic hand tremor may be initially misdiagnosed as PD-related tremor and, along with rest tremor associated with ET, may be responsible for scans without evidence of dopamine deficiency (SWEDDs) (Schneider et al., 2007; Bain, 2009; Schwingenschuh et al., 2010; Stoessl, 2010). When 25 tremulous SWEDDs patients were compared to 25 tremor-dominant PD patients, the former group lacked true bradykinesia, they had evidence of dystonia, and head tremor, whereas reemergent tremor, true fatiguing or decrement, good response to dopaminergic drugs, and presence of nonmotor symptoms favored PD (Schwingenschuh et al., 2010). Whether the hand tremor that is seen in about 25% (10–85%) of patients with cervical dystonia represents an enhanced physiologic tremor, ET, dystonic tremor, or some other form of postural tremor is unknown (Jankovic et al., 1991; Deuschl et al., 1997). Although the frequency of ET and the limb tremor in patients with cervical dystonia are similar, the tremor amplitude in patients with dystonia is smaller and the tremor is more irregular, suggesting that the two types of tremors, while similar, arise from different types of oscillators (Shaikh et al., 2008). The observation of significant overlap in association between variants in LINGO1 and ET and PD (see below) provides support for the genetic association between the two common movement disorders (Vilariño-Güell et al., 2010a).
The clinical heterogeneity of ET suggests that there may be different subtypes. Indeed, Louis and colleagues (2000b) found that patients with older onset (>60 years) and those without head tremor progressed more rapidly than did patients with young-onset tremor and those with head tremor. They also later found that head tremor was present four times more frequently in women than in men (Louis et al., 2003; Hardesty et al., 2004). Using the medical records linkage system of the Rochester Epidemiology Project, the authors identified ET patients who also had an autopsy report and found that women with ET were six times more likely to develop head tremor than men (Hardesty et al., 2004). The presence of jaw tremor, seen in 7.5–18.0% of patients with ET, has been found to be associated with older age at onset, more severe action tremor in arms, and the presence of head and voice tremor (Louis et al., 2006a). In a study of 34 patients with voice tremor, 93% were female and the voice tremor typically began in the seventh decade (62.9 ± 15.0 years) (Sulica and Louis, 2010). More than a third had a first-degree relative with tremor and more than a quarter reported a beneficial effect of ethanol. In this study only 11 (32.3%) were aware of an arm tremor and 10 (29.4%) had been misdiagnosed as spasmodic dysphonia. Only 56% of treated patients found botulinum toxin helpful and the response was often incomplete. Jaw tremor was also significantly associated with rest tremor, suggesting that some patients with ET and jaw tremor may convert to PD. Besides ET and PD, jaw tremor may be a manifestation of dystonia (Schneider and Bhatia, 2007). Lower extremity tremor is usually mild or asymptomatic (Poston et al., 2009).
The ongoing debate as to whether ET is a monosymptomatic or heterogeneous disorder or phenotypic manifestation of multiple entities will probably not be resolved until disease-specific physiologic, genetic, or other biologic markers are identified (Schrag et al., 2000; Elble, 2002; Jankovic, 2002; Elble and Tremor Research Group, 2006; Louis, 2009; Deuschl and Elble, 2009). One hypothesis is that the various nonmotor signs linked to ET could be secondary to “abnormal neuronal oscillation” (Deuschl and Elble, 2009), but his would not explain the heterogeneous presentation of ET. Also, the proposed classification of ET into “hereditary” (unequivocal family history), “sporadic” (no immediate family member with ET), and “senile” (onset after age 65) is too artificial and not easily applicable. Some studies have suggested that there is an association between ET and parkinsonism (Jankovic et al., 1997; Yahr et al., 2003; Shahed and Jankovic, 2007; Fekete and Jankovic, 2011), but other studies have not found a link (Adler et al., 2011) (Fig. 18.2). Differentiation between ET and PD is critical particularly in early stages since ET has been found to be erroneously treated with anti-PD drugs in 12.4% of 402 community cases re-evaluated by a movement disorder specialist (Meara et al., 1999). Postural tremor, similar to ET, has been reported to occur in as many as 93% of patients with PD and to correlate with the ipsilateral rest tremor but not with age at onset or disease duration (Louis et al., 2001e). Furthermore, in 22 patients with PD with family history of ET, 90% (20 of 22) had a tremor-predominant subtype of PD, suggesting that “these patients have inherited a genetic susceptibility factor for tremor, which affects the motor phenotype of PD” (Hedera et al., 2009). Phenomenologically similar to ET, the postural tremor of PD has been linked by some investigators to coexistent ET (Geraghty et al., 1985; Jankovic, 1989; Jankovic et al., 1997; Jankovic 2000). Others, however, believe that the coexistence of the two disorders simply “represents a chance occurrence of two common diseases” (Pahwa and Koller, 1993). On the basis of an analysis of 678 patients diagnosed as having ET, some by movement disorder specialists and others by private practice neurologists, 6.1% were found to have concomitant PD, and 6.9% had coexisting dystonia (Koller et al., 1994). The authors concluded that “the frequency of PD in ET is more than would be reported in the general population.” In the Neurological Disorders in Central Spain (NEDICES) study, a longitudinal, population-based study of 3813 people (mean age 73.4 ± 6.6 years), after a median of 3.3 years, 12 (5.8%) of 207 ET cases developed parkinsonism compared with 56 (1.6%) of 3606 controls, with adjusted relative risk (RR) of 3.47 (95% confidence interval 1.82–6.59; P < 0.001) (Benito-León et al., 2009b). Six (3.0%) of 201 ET cases developed incident PD versus 24 (0.7%) of 3574 controls, with adjusted RR of 4.27 (95% confidence interval 1.72–10.61; P = 0.002). The authors concluded that “Patients with ET were four times more likely than controls to develop incident PD during prospective follow-up.”
The coexistence of ET and PD may be difficult to recognize because once a patient develops PD, the postural tremor is usually attributed to the disease, and it is therefore difficult to diagnose ET in a patient who already has symptoms of PD (Shahed and Jankovic, 2007; Louis, 2009; Fekete and Jankovic, 2011) (Fig. 18.2). While rest tremor may be observed in patients with advanced ET, it may also be the initial manifestation of coexistent PD (Shahed and Jankovic, 2007; Fekete and Jankovic, 2010). The “postural tremor” that is seen in many patients with PD may represent an enhanced physiologic tremor (Forssberg et al., 2000), coexistent ET (Geraghty et al., 1985; Jankovic, 1995), or a reemergent classical rest tremor (Jankovic et al., 1999) with the same frequency and clinical characteristics as the typical rest tremor. This reemergent tremor is also often exacerbated during walking. In contrast to ET, which is seen immediately when patients outstretch their arms, the reemergent tremor of PD usually appears after a latency of several seconds (Jankovic et al., 1999). Furthermore, this PD-related tremor often responds to levodopa, whereas the postural tremor of ET does not (Kulisevsky et al., 1995). It is actually this action tremor that seems to correlate with motor disability rather than the typical rest tremor, which correlates chiefly with social handicap (Zimmermann et al., 1994). The clinical characteristics, however, may not always reliably differentiate between the two types of postural tremor (Henderson et al., 1995). We found a higher frequency of the 263 bp allele of the NACP-Rep1 polymorphism not only in patients with PD (odds ratio: 3.86) but also in patients with ET (odds ratio: 6.42), but not in patients with Huntington disease, supporting a genetic link between PD and ET (Tan et al., 2000). Further evidence that ET and PD may be related is the observation that patients with ET have an olfactory deficit that is similar, although milder, than that noted in patients with PD (Louis et al., 2002; Louis and Jurewicz, 2003; Djaldetti et al., 2008). In one study, however, there was no difference in results of olfactory testing between ET patients and controls (Shah et al., 2008). We and others (Gimenez-Roldan and Mateo, 1991) have noted that patients with ET seem to have a higher propensity toward neuroleptic-induced parkinsonism than do patients without ET, although a formal epidemiologic study is needed to confirm this clinical observation. To examine an ET–PD relationship, we described 22 patients with childhood-onset ET who later developed PD (Shahed and Jankovic, 2007; Fekete and Jankovic, 2011). Of 11 patients reporting asymmetric ET, PD symptoms began on the same side as the more severe ET tremor in 10 (90.9%, χ2 = 0.66, P = 0.024), with 68.2% reporting change in tremor as their first PD manifestation. These findings, supported by another study (Tan et al., 2006), suggest that in some patients, childhood ET evolves into adult tremor-dominant PD, explaining the coexistence of ET and PD within the same patient and family. It has been postulated that ET-related gene mutations may predispose some patients to subsequent development of PD. In a study of 53 patients with ET–PD combination, compared to 53 PD and 150 ET patients, the side of the greatest initial ET severity corresponded to the side of the greatest PD severity (Minen et al., 2008). In another study involving 13 patients who presented originally with asymmetrical postural tremor and no rest tremor for at least 10 years (mean: 19.2 years) and were initially diagnosed with ET, all patients subsequently developed evidence of PD (Chaudhuri et al., 2005). The onset of levodopa-responsive PD was manifested by rest tremor for a mean of 2.5 years before final presentation in the clinic. Furthermore, five patients who had β-CIT single photon emission computed tomography (SPECT) all showed reduced uptake in the contralateral striatum. It is not clear whether these patients with a long-standing history of asymmetrical postural tremor have PD at onset, whether patients with unilateral postural tremor (isolated tremor) or asymmetrical postural tremor (atypical ET) who later develop PD represent an overlap between ET and PD, or whether this type of postural tremor is an early marker for PD (Grosset and Lees, 2005).
In a population-based study (981 first-degree relatives of 162 patients with PD and of 838 first-degree relatives of 147 controls), the risk of ET was significantly increased for relatives of patients with onset of PD (P = 0.006) (Rocca et al., 2007). Also, in a referral-based sample (981 first-degree relatives of 162 patients with PD and of 838 first-degree relatives of 147 controls), the risk of ET among relatives increased with younger onset of PD in patients (P = 0.001) and was higher in relatives of PD patients with the tremor-predominant or mixed form when compared with relatives of patients with the akinetic-rigid form, and in men compared with women. The authors concluded that “These findings suggest that PD and ET may share familial susceptibility factors.” In a case-control study of 600 subjects evaluated for tremor, ET was significantly more frequent in patients with PD (12/204, 5.9%) compared to diseased controls (2/206, 1%) and healthy controls (1/190, 0.5%) (Tan et al., 2008). The authors concluded that “PD patients were 5–10 times more likely to have ET compared diseased and healthy controls.”
In addition to genetic factors, there may be environmental factors that determine the occurrence of ET and its relationship to PD. For example, heavy cigarette smoking has been associated with lower risk of PD and ET (Louis et al., 2008a). Although some studies have concluded dementia is more frequent in patients with ET than in controls (Bermejo-Pareja et al., 2007), and one study reported the adjusted odds ratios to vary between 1.64 and 1.84 (Thawani et al., 2009), a relationship between ET and Alzheimer disease has not been established (Elble et al., 2007a).
The possibility of additional cochlear involvement in ET is supported by the observation of high occurrence of partial or complete deafness in patients with ET (Ondo et al., 2003). Among 250 patients with ET, 42 (16.8%) patients wore hearing aids, compared to only 2 of 127 (1.6%) PD patients and 1 of 127 (0.8%) controls (P < 0.0001). Pure tone audiometry demonstrated age-dependent higher-frequency loss among patients with ET as compared to the general population. High risk of hearing loss among patients with ET has been confirmed by other studies (Benito-León et al., 2007). The combination of ET, sensorineuronal hearing loss, and early graying has been suggested to be a unique disorder, separate from Waardenburg syndrome (Karmody et al., 2005). Although mental functioning is usually intact in patients with ET, detailed testing of cognitive performance has found some subtle abnormalities on tests of verbal fluency, naming, mental set-shifting, verbal working memory, and other tests of cognitive function (Benito-León et al., 2006a) and elderly patients with ET may possibly have an increased risk of dementia compared with those without ET (Benito-León et al., 2006b). Furthermore, depression has been found to occur in about a third of the patients with ET, almost as frequently as in PD (Lombardi et al., 2001; Miller et al., 2007). These deficits have been interpreted as suggesting involvement of frontocerebellar circuits. In a cross-sectional study of personality, patients with ET were found to have a tendency to have increased levels of pessimism, fearfulness, shyness, anxiety, and easy fatigability, but none of these traits correlated with the severity of the tremor (Chatterjee et al., 2004).
ET appears to be a heterogeneous disorder, as is suggested by the multiple gene loci that have so far been identified and by the frequent association with other disorders, such as parkinsonism, dystonia, and myoclonus (Jankovic et al., 1997; Jankovic, 2002; Yahr et al., 2003; Deng et al., 2006b; Shahed and Jankovic, 2007) (Fig. 18.2). Postmortem studies of patients with ET have not provided evidence for nigrostriatal pathology in ET, but patients who had a diagnosis of PD at time of death (even though ET might have preceded the onset of PD) would have been excluded from these clinical-pathologic studies (Jankovic, 2004; Rajput et al., 2004). Furthermore, there is indirect evidence suggesting nigrostriatal impairment in some patients with ET. We found that relatives of patients with PD have at least a 2.5 times higher (those with the combination of ET–PD: 10 times higher) frequency of tremor than normal controls, providing additional support for the association of ET and PD (Jankovic et al., 1995a). Furthermore, about 20% of patients with ET have a rest tremor that has the clinical and physiologic characteristics of PD tremor (Cohen et al., 2003). However, when 9 brains of patients who exhibited advanced ET and upper extremity rest tremor (without any other evidence of parkinsonism) were examined with alpha-synclein staining, only two had Lewy bodies in the dorsal vagus nucleus and locus ceruleus, but none had Lewy body-containing neurons and/or Lewy neurites in the basal ganglia (Louis et al., 2011). Although this pathological study suggests that rest tremor in patients with ET is not associated with PD pathology, 19 of 24 (80%) patients with ET and rest tremor without other parkinsonian features had abnormal DAT uptake on DaTscan, particularly in the putamen (deVerdal et al., 2011). Similarly, a fourfold increase in prevalence of isolated tremor among relatives of patients with PD as compared to controls was found by Payami and colleagues (1994). Interestingly, among 196 twins with postural or kinetic tremors, Tanner and colleagues (2001) found that 137 had PD or had a twin with PD.
Imaging studies have been helpful in providing insight into the relationship between ET and PD. A 10–13% reduction in 18F-dopa uptake in the striatum of patients with ET as compared to controls (Brooks et al., 1992) suggests a physiologically important compromise of the dopaminergic system in patients with ET (Jankovic et al., 1993). Furthermore, 18F-dopa uptake constants (Ki) in 5 of 32 asymptomatic relatives of patients with PD who had isolated postural tremor were reduced on average by 23% (P < 0.001) (Piccini et al., 1997). The mean Ki for the other 27 asymptomatic relatives was decreased by 17% (P < 0.001). Using 123I-IPT SPECT to image the striatal dopamine transporter, Lee and colleagues (1999) found the mean bilateral uptake in nine patients with isolated postural tremor (ET) to be slightly lower than that in normal control subjects (3.60 vs. 3.80), but this did not reach statistical significance. Six other patients in whom rest tremor developed 4–18 years (mean: 11.5 ± 6.7) after the onset of postural tremor without other parkinsonian features, however, had a significant reduction in the dopamine transporter compared to normal controls (2.61 vs. 3.83, P < 0.05) but lower than PD patients (1.97 contralateral and 2.35 ipsilateral). They concluded that some patients with postural tremor may acquire rest tremor in association with mild substantia nigra neuronal loss. Although the majority of ET patients have normal dopamine transporter (DAT) SPECT, some cases may start with isolated postural tremor, phenomenologically identical to ET, and later develop PD. In one study the mean latency between the onset of asymmetrical postural tremor and PD was 19.2 years whereas the mean latency between onset of rest tremor and PD was 2.5 years (Chaudhuri et al., 2005). In one study of 61 subjects presenting with “isolated atypical tremors defined as unilateral either postural, resting or mixed” followed at baseline and at mean 28.4 ± 7.2 months with 123I-FPCIT SPECT, those (n = 25) with normal baseline scan had only tremor at follow-up, and of the 36 with abnormal baseline scan, 23 (64%) developed PD, while the remaining patients had only tremor (presumably ET) (Ceravolo et al., 2008). They suggested that term “isolated tremor with dopaminergic presynaptic dysfunction” is used for the patients with unilateral or asymmetrical tremor with abnormal DAT SPECT. Whether the use of 123I-FPCIT SPECT in differentiating ET from PD is cost-effective is not clear, although one Italian study suggested some cost savings when using this diagnostic tool (Antonini et al., 2008). In one study FP-CIT SPECT showed that the pattern of dopaminergic loss over time is different between ET and PD, but both disorders exhibit impairment of DAT in the caudate nucleus (Isaias et al., 2010). Although this and other imaging studies reported by the same group (Isaias et al., 2008) provide important insights into the selective caudate dopaminergic deficit as a possible link between the two common disorders, some studies have reported that DAT SPECT remains normal over time in patients with mixed tremor (a combination of postural and rest tremor) (Arabia et al., 2010). Since medial substantia nigra (that predominantly projects to the caudate nucleus) is particularly involved in the tremor-dominant PD and is associated with more caudate loss of DAT, it is possible that tremor-dominant PD and ET share a selective dopaminergic loss in the caudate nucleus. This, in turn, may lead to a dysfunction of the caudate-thalamic pathway and disinhibition of the thalamic autorhythmic pacemakers, clinically expressed as tremor. Indeed, β-CIT SPECT, which mainly reflects serotonin transporters, is lower in the thalamus of patients with tremor-dominant PD as compared to those with non-tremor PD (Caretti et al., 2008). In addition to thalamus, the caudate also projects to the inferior olive and cerebellum, both implicated in the pathophysiology of ET, and supported by growing evidence or cerebellar pathology in ET. The clinical overlap between the two disorders undoubtedly contributes to the 10–15% frequency of patients diagnosed with mild PD who have SWEDDs (Schneider et al., 2007; Bain, 2009; Schwingenschuh et al., 2010; Stoessl, 2010).
A relationship between ET and nigral degeneration is supported by the finding of hyperechogenicity of substantia nigra on midbrain sonography in 16% of 44 ET patients as compared to 3% of 100 controls and 75% of 100 patients with PD (Stockner et al., 2007). Although not demonstrated by all studies (Doepp et al., 2008; Budisic et al., 2009), the slightly increased hyperechogenicity of the substantia nigra on midbrain sonography provides further support for the notion that some ET patients may later develop parkinsonism.
Although the relatively frequent coexistence of ET and dystonia supports the notions that there is a pathogenetic link between the two disorders, linkage analysis has excluded the dystonia (DYT1) gene on chromosome 9 in hereditary ET (Conway et al., 1993; Dürr et al., 1993). This suggests that the genes for these two disorders are on separate loci or that the relationship between the two disorders is physiologic rather than genetic. Münchau and colleagues (2001) studied 11 patients with classic ET and compared them to 19 patients with cervical dystonia and arm tremor. They found that the latency of the second agonist burst during ballistic wrist flexion movements was later in ET patients than in those with arm tremor associated with cervical dystonia. Furthermore, the latter group had a greater variability in reciprocal inhibition than the ET group. Patients with normal presynaptic inhibition had simultaneous onset of their arm tremor with onset of their cervical dystonia (mean age: 40 years), whereas patients with reduced or absent presynaptic inhibition had an earlier age at onset (mean 14 years), and the interval between the onset of the tremor and the onset of cervical dystonia was longer (mean: 21 years). This suggests that the mechanisms of arm tremor in patients with ET and cervical dystonia are different. The association between ET, dystonia, and PD is suggested by reports of families with manifestations of these three disorders in different or same members of the families (Jankovic et al., 1997; Yahr et al., 2003).
ET-like tremor has been described in patients with hereditary myoclonus and with hereditary motor-sensory neuropathy (sometimes referred to as Roussy–Levy syndrome) (Cardoso and Jankovic, 1993). ET-like tremor occurs in other genetic diseases, the study of which may provide important insights into possible genetic heterogeneity in families with clinically similar tremor. For example, postural tremor similar to that seen in ET has been reported in patients with Kennedy disease, also called X-linked recessive spinal and bulbar muscular atrophy, which is caused by a mutation characterized by expansion of CAG repeats in the gene on the X chromosome (Sperfeld et al., 2002). ET may also be associated with higher-than-expected frequency with restless legs syndrome (Ondo and Lai, 2006). The validity and meaning of such associations, however, are disputed, and the controversies are not likely to be resolved until a disease-specific marker (e.g., an ET-linked genetic locus) is identified. A diagnostic marker for ET would also help to resolve the question as to whether site-, position-, and task-specific tremors, such as primary handwriting tremor and orthostatic tremor, are distinct entities or whether these tremors represent clinical variants of ET (Rosenbaum and Jankovic, 1988; FitzGerald and Jankovic, 1991; Britton et al., 1992b; Danek, 1993; Soland et al., 1996b; Sander et al., 1998) (Table 18.5). It is still not clear whether primary writing tremor is a variant of ET, a type of focal dystonia such as writer’s cramp, or a separate nosological entity (Hai et al., 2010; Quinn et al., 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree