CHAPTER 308 Tumors of the Craniovertebral Junction
The craniovertebral junction is a biomechanical and anatomic unit that comprises the clivus, foramen magnum, and upper two cervical vertebrae. The neoplasms that arise within the structures are osseous in nature or extensions from the soft tissue that surround the craniovertebral junction, or they are neoplasms that arise from the neural structures contained within the bony anatomy.1–4 The diagnosis of such lesions has been greatly facilitated by modern neurodiagnostic imaging.
There is no single symptom or neurological finding that is pathognomonic for a lesion in this location.5,6 Because of the generous size of the subarachnoid spaces at the cervicomedullary junction, symptoms arise only after a lesion has achieved large proportions. These patients have a fluctuating neurological course, and an erroneous diagnosis is common owing to the anatomic complexities of the decussation of the sensory and motor tracts.1,6
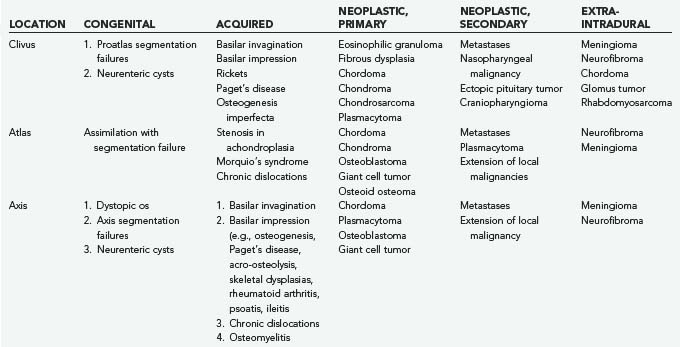
The first systematic evaluation of foramen magnum tumors was performed by Elsberg and Strauss.7 Several authors have since reported extra-axial lesions affecting the region, such as meningiomas and neurinomas.8–12 Osseous neoplastic involvement of the craniovertebral junction may be due to chordoma, chondrosarcoma, plasmacytoma, osteoblastoma, fibrous dysplasia, metastatic tumor, and giant cell tumor. Table 308-1 summarizes my experience.
Common Clinical Manifestations of Craniovertebral Junction Tumors
Tumors of the craniovertebral junction, whether extracranial with secondary involvement of the intracranial and intraspinal structures or primarily intracranial with secondary extension into the spinal canal, have characteristics that reflect compression of neighboring structures or traction. They also may have distal effects such as hydrocephalus, syringohydromyelia, and vascular compromise.9,13,14 Chordoma often involves the cranial base and upper cervical spine extensively and may be associated with only minimal complaints of headache and neck pain for several years. Unfortunately, this hiatus is followed by a rapid progression of brainstem and cervical spinal cord dysfunction that brings the lesion to light. In the report of Meyer and coworkers, the time from the onset of symptoms to the diagnosis of extramedullary tumor at the foramen magnum was 2.5 years.2
The clinical presentation of craniovertebral junction tumors can be divided into intracranial lesions, “straddle lesions,” and those affecting the high cervical spinal cord.7 The effects of vascular compromise and alterations of cerebrospinal fluid (CSF) circulation add to the constellation of symptoms and signs.
Patients with intracranial lesions present with involvement of the lower cranial nerves, brainstem dysfunction, and occasionally cerebellar symptoms. Patients with straddle lesions have a paucity of cranial nerve dysfunction and a predominance of high cervical myelopathy. High cervical lesions do not produce cranial nerve and cerebellar signs, except for involvement of the spinal accessory nerve and sometimes the descending tracts of the trigeminal nerve and the lower decussations of the motor and sensory tracks.15
The most common presentation is pain referred to the second cervical dermatome.1 The head is held flexed, and the condition may resemble torticollis. The pain is described as an aching sensation that is aggravated by neck and head motion and referred to the suboccipital region. Unfortunately, the symptom of pain alone may predate other clinical findings for many years. Paresthesias or dysesthesias of the face, hands, and limbs are frequently reported. An abnormal cold sensation of the lower extremities was described by Elsberg and Strauss7 and by Beatty13 as being pathognomonic of lesions of the high cervical cord. Most frequently, pain and temperature sensation is affected, followed by loss of joint sensation. This finding is seen in the upper extremities and may then proceed clockwise around the limbs. A suspended sensory loss with patches of preservation of sensation in the upper extremities may confuse the presentation. “Dissociated” sensory loss has been described in about one fourth of extracranial lesions of the cervicomedullary junction, even though this finding has been considered to reflect an intramedullary process.2
Spastic weakness of the extremities is a prominent feature in patients with tumors in this region. The weakness may begin in the ipsilateral limb and progress to the lower limb of the same side, followed by weakness of the contralateral lower limb; finally, weakness becomes apparent in the contralateral upper limb. This distinct progression of motor symptoms is an important characteristic of lesions of the cervicomedullary junction.15 Localized wasting of the intrinsic muscles of the hand may develop ipsilateral to the lesion. Taylor and Byrnes postulate venous stagnation of the anterior horn cells and the lower cervical cord as a result of decreased venous drainage, which typically occurs rostral to the lower portion of the cervical spinal cord.16 Other proposed mechanisms include anterior spinal artery compression, hydromyelia secondary to CSF obstruction, venous obstruction with spinal cord edema, and spinal cord rotation with contralateral traction.
A tumor at the foramen magnum may produce a mixture of upper motor neuron findings in the upper and lower extremities. This pattern reflects the pyramidal decussation that begins just below the obex and ends near the uppermost cervical spinal cord. The more medial fibers of the pyramidal tract carry impulses to the upper extremities and cross superior to the lateral fibers that serve the lower extremities. Similarly, the sensory decussation of the medial lemniscus may produce a varied pattern of sensory abnormalities.17 Thus, a tumor situated at the ventral aspect of the cervicomedullary junction can cause sensory aberrations in the lower extremities first. The syndrome of cruciate paralysis has been associated with trauma as well as tumors with basilar invagination.18
Transient symptoms may be due to both vascular changes and instability at the craniovertebral junction.1,19 Such symptoms can manifest as paralysis, paresthesias, drop attacks, and vertebrobasilar syndromes such as migraine and visual loss in the homonymous visual fields. Cranial nerve palsies may be the result of nuclear involvement in the brainstem, traction, compression of the subarachnoid segments, or interosseous disease.1,15 The most common cranial nerves affected are the vagus, glossopharyngeal, and hypoglossal. Their involvement leads to dysphagia, slurred speech, and repeated episodes of aspiration into the tracheobronchial tree, resulting in pneumonia and weight loss. Tumors of the upper cervical canal can present with involvement of the spinal root of the accessory nerve, manifesting as torticollis and weakness of the trapezius and sternocleidomastoid muscles. About 15% to 20% of patients develop tinnitus, vertigo, and hearing loss related to involvement of the vestibulocochlear nerve.15
The differential diagnoses considered most often by Meyer and coworkers in their initial evaluation of 102 documented cases of benign extramedullary tumors of the foramen magnum included cervical spondylosis (25%), multiple sclerosis (18%), syringomyelia (17%), intramedullary tumor (15%), Chiari’s malformation (5.5%), and carpal tunnel syndrome (5.5%).2 Other erroneous diagnoses in patients with lesions at the craniovertebral junction are intramedullary tumors of the brainstem and upper cervical cord, amyotrophic lateral sclerosis, and subacute combined degeneration. Cervical spondylosis may be difficult to differentiate when it coexists. The presentation of restless legs syndrome in patients with craniovertebral compressive pathology has been well documented by Glasauer and Egnatchick.20
Neurodiagnostic Imaging
The complex anatomy and pathology of tumors in this region demand precise definition and depiction of the tumor’s extent and its relationship to the vital structures of the brainstem and spinal cord, lower cranial nerves, and vascular structures. Thus, complementary multimodality imaging includes plain radiography, magnetic resonance imaging (MRI), magnetic resonance angiography, computed tomography (CT), and three-dimensional CT angiography. The sensitivity of MRI is greatly enhanced by the addition of intravenous gadolinium and by performing separate magnetic resonance venography and angiography.5
Cerebral angiography is useful in understanding the dynamics of collateral circulation and tumor vascularity. Temporary balloon occlusion is a means of assessing a patient’s tolerance of vascular occlusion of the carotid or vertebral circulation before surgery.15 Such information is especially useful when lesions are encased by tumor. These two tests provide information about the resectability of difficult lesions.
Electrophysiologic Tests
Intraoperatively, we monitor somatosensory brainstem evoked potentials and perform facial electromyography when lesions extend to the cerebellopontine angle and when using the preauricular infratemporal fossa approach.15,21,22 The intraoperative evaluation of cranial nerve IX and cranial nerve X function is accomplished by placing electrodes in the soft palate and against the true vocal cords, incorporating these latter electrodes in the endotracheal tube for the anesthetic. Hypoglossal nerve electromyography supplements the evaluation with an electrode placed directly into the tongue. Brainstem monitoring still yields a significant number of false-negative and false-positive results, but improved techniques make these adjuncts useful in the intraoperative assessment of the function of the cervicomedullary junction.
Surgical Approaches and Decision Making
The goal of treating benign osseous pathology differs from that of treating malignant disease, in which complete excision is the objective. Benign lesions create a space among the neurovascular structures, thereby allowing surgical debulking and resection “from within.” Malignant disease, however, requires a much more radical resection with clean margins. In most instances, benign lesions such as chordomas are radioresistant; hence, gross total resection should be the aim. Craniovertebral stability, both before and after operative intervention, must be considered in the development of approaches.14,19,23 Thus, the factors that influence the specific treatment of tumors arising in this region are (1) mechanism of compression and direction of encroachment, (2) whether the lesion is benign or malignant, (3) whether the lesion has an associated vascular or intramedullary component (e.g., syringomyelia), (4) craniospinal stability, and (5) patient age. Lesions of the craniovertebral junction do affect the pediatric population, although to a lesser extent than they affect adults.3,5,24 In children, the potential for growth, concerns about stability, and the patient’s size are critical. From this perspective, midface growth centers are the nasal septum and the pterygoid plates. Hence, in a child, a transpalatal approach to the clivus and the sella would be considered before a sublabial transsphenoidal or a maxillary drop-down procedure, with the goal of avoiding damage to the growth centers.25
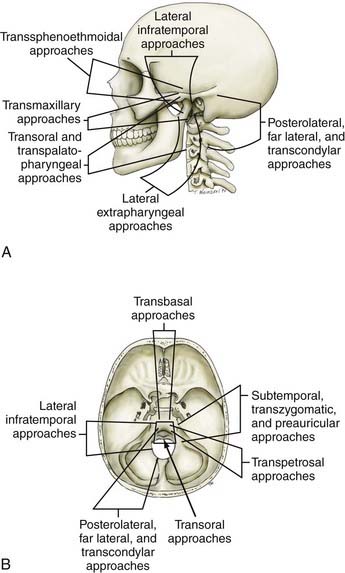
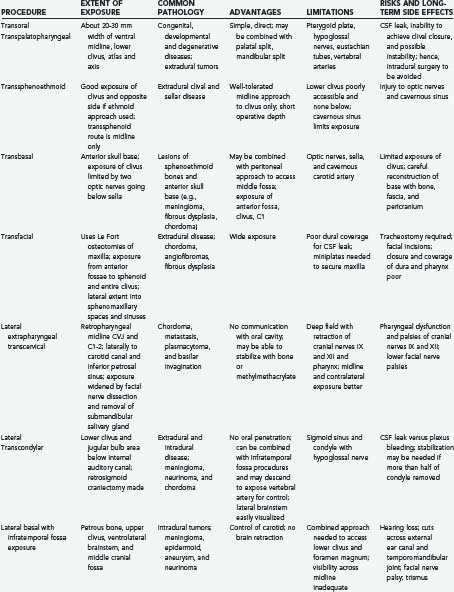
Advances in neurodiagnostic imaging and microsurgical instrumentation have allowed the development of extensive surgical approaches based on an understanding of the complex anatomy, the craniovertebral dynamics, and the site of encroachment. Consequently, the entire circumference of the foramen magnum is within the neurosurgeon’s reach (Fig. 308-1). However, several surgical limitations and considerations must be appreciated when designing an approach to the craniovertebral junction (Table 308-2). The most frequently used anterior route is the transoral-transpalatopharyngeal approach.1,25–27 Laterally, this approach is limited by the pterygoid plates, the hypoglossal canal, the eustachian tubes, and the width between the vertebral arteries. The limitation imposed by the pterygoid plates can be overcome by using a transmaxillary route with down-fracture of the maxilla and an extended maxillotomy if needed.1,28 A combined exposure with mandibulotomy and midline glossotomy lowers the midline exposure to C5.5,29,30 The disadvantages of the ventral approaches are overshadowed by their easy access, ability to cross the midline, and vertical extensions.31,32 The ventral and dorsal midline approaches permit the anterior and posterior 90 degrees of the craniovertebral junction to be scanned, respectively.
As a general rule, midline extra-axial tumors are best approached in an extra-axial fashion without retracting the brain or violating the dura. A lateral avenue should be used for tumors that are situated laterally.33–35 The transsphenoidal-transsphenoethmoidal approach provides a shallow operative depth and is well tolerated as a route to the upper two thirds of the clivus.36–38 This exposure provides better access to the contralateral side; hence, at times, a bilateral approach may be necessary. The transfacial routes provide wide access to the anterior and midline skull base and include the ventral aspect of the clivus.39,40 This approach is ideal for treating extradural lesions such as chordomas, angiofibromas, and fibrous dysplasias. Dural coverage must be provided by vascularized muscle flaps obtained from the temporalis muscle.
The transbasal approach provides access to the anterior skull base. Unless combined with removal of the supraorbital bar, this approach is limited by the distance between the two optic nerves and the need to work beneath the sella turcica.23 Nonetheless, we have used this approach in several patients and have achieved good reconstruction of the floor.
A lateral extrapharyngeal route is effective and safe for reaching the upper cervical spine.41 At the clivus, however, access becomes difficult because of the pyramidal narrowing of the exposure at the depths of the wound. In an effort to correct this problem, we reroute the facial nerve with upward displacement of the angle of the mandible,25 thereby exposing the lower clivus. We believe that this approach should be limited to metastases, chordomas, and plasmacytomas that affect the axis and atlas vertebrae. Its use for treatment of basilar invagination and intradural pathology is limited.
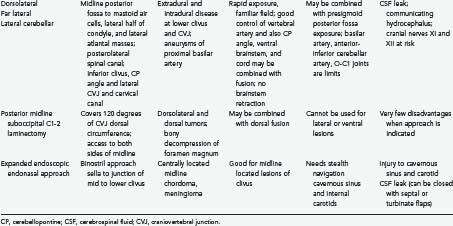
The true lateral-transcondylar approach to the ventral aspect of the lower brainstem and clivus, as well as the upper cervical cord, demands resection of a portion of the lateral atlantal mass and the occipital condyle. Exposure of the lower clivus and the ventral brainstem is enhanced when combined with a retrosigmoid craniectomy.34 It is useful in the treatment of both extradural and intradural lesions such as chordomas, meningiomas, and neurofibromas.4,14,33 When combined with infratemporal procedures, it allows both anterior and posterior extensions that overcome the limitations of the sigmoid sinus and the hypoglossal nerve.1,34,42 The risk for CSF leak and the possibility of destabilization are high. Troublesome bleeding is routinely encountered from the paravertebral venous plexuses.
The posterolateral route has been recognized for many years. It enables scanning of the foramen magnum to the 90 degrees available with the posterior midline approach as well as the 90 degrees available with the lateral transcondylar approach.1,43 The latter uses a standard midline posterior exposure with a lateral cerebellar approach and includes partial resection of the mastoid process and the posterior third to half of the occipital condyle to provide exposure of the jugular bulb and the medial aspect of the lateral atlantal mass. This provides access to the vertebral artery, which can be rerouted and displaced from the foramen transversarium, thus allowing for control from the C2 level upward. The ability to create a fusion makes this an ideal approach for posterior, lateral, and ventrolateral lesions. Thus, the entire circumference of the foramen magnum is accessible.
The expanded endonasal endoscopic approach is a valid, minimally invasive alternative for treatment of centrally located clivus chordomas or any such lesions or could be an adjunct for the central part of a lesion with lateral extension.36,44–46 A standard nasal access is used with the binostril approach, with the endoscope typically positioned in the right superior nasal cavity by one surgeon who is responsible for maintaining the visual field. This arrangement allows a second surgeon to use both hands and an instrument in each nostril for the resection of the tumor. Dissection of both middle turbinates can expose a large working field, although this is not essential. The mucoperiosteal flaps from the middle turbinate can be used to close off a CSF leak.38 The medial wall of the clival carotids represents the lateral limits of the clival exposure. The floor of the sphenoid is further drilled to get access to the mid and lower clivus. Resection of medial pterygoid process can be performed for exposure of lateral extension of the lower part of the tumor. A total ethmoidectomy can augment exposure into the upper clivus. Closure is performed with multilayered reconstruction technique. In the series by Dehdashti and associates,46 12 patients underwent such operation over a 3-year span. There were four CSF leaks. One patient developed hydrocephalus and one new deficit with clot and one pneumocephalus. Cranial deficits improved over time. A gross resection was accomplished in 7 of the 12 patients. In the series by Frank and associates,45 nine chordomas were approached in this fashion, with a total removal in three. An internal carotid injury occurred in one patient, with two persistent CSF leaks.
Extradural Tumors of the Craniovertebral Junction
Clival and Craniovertebral Junction Chordomas
Chordomas are rare, aggressive, locally destructive tumors of presumed notochordal origin that arise along the vertebral axis and show a proclivity for the spheno-occipital and sacral regions.1,9,37,47 Chordomas of the clivus and craniovertebral junction are the most common extradural neoplasms in this region. The overall incidence of chordomas is 0.2 to 0.5 per 100,000 persons per year, and they account for about 0.15% of all intracranial tumors. About 25% of chordomas occur at the base of the skull, arising from the clivus. Although usually midline, the notochord may have distal projections that extend to the clinoid processes of the petrous bones. Most patients experience symptoms referable to the tumor for more than a year before diagnosis.
Pathology
Chordomas have been divided into classic chordomas, chondroid chordomas, and atypical chordomas.48 Classic chordomas are lobulated, pinkish gray, gelatinous tumors that infiltrate bone but may appear grossly as somewhat demarcated; they account for 80% to 85% of all chordomas. Histologically, they exhibit a variable mix of sheets and cords or clusters of small polygonal cells with eosinophilic cytoplasm and hyperchromatic nuclei. A myxoid matrix is present. Cytologic atypia is absent or minimal.
A subpopulation, the chondroid chordoma, arises in the spheno-occiput and exhibits cartilaginous differentiation. Some authors dispute the existence of chondroid chordomas, preferring to regard these cartilage-containing neoplasms as chondrosarcomas. Chondroid chordomas have a more indolent clinical course; the survival rate is 15.8 years, compared with 4.1 years for typical classic chordomas.47,48 Chondroid chordomas account for 5% to 15% of all chordomas.
Atypical chordomas have a sarcomatoid appearance, with round cells and epithelial or spindle cells present with large areas of necrosis. These solid tumors are aggressive and account for 1.3% to 8% of all chordomas. In the series by Heffelfinger and coworkers, only one patient with an atypical chordoma survived more than 10 years, whereas almost 50% of those with chondroid chordomas survived more than 10 years.48 The frequency of mitotic figures, nuclear pleomorphology, and hyperchromatism does not appear to affect the ultimate outcome.49,50 In rare circumstances, chordomas may dedifferentiate into malignant chondrosarcomas, fibrosarcomas, and even osteosarcomas.51
The immunohistochemical profile of reactivity with antibodies to vimentin cytokeratin, epithelium membrane antigen, and S-100 protein tend to distinguish chordomas from other sarcomatoid round cell or myxoid neoplasms.49,50 Chondrosarcomas are negative for cytokeratin, epithelium membrane antigen, and carcinoembryonic antigen. Vimentin and S-100 protein are present in both chondrosarcomas and chordomas. Immunostaining from keratin has no prognostic value regarding the aggressiveness of the tumor. Chondrosarcomas have been lumped with chordomas because of supposed parallel lines of occurrence, location, and aggressive behavior.
Imaging Characteristics
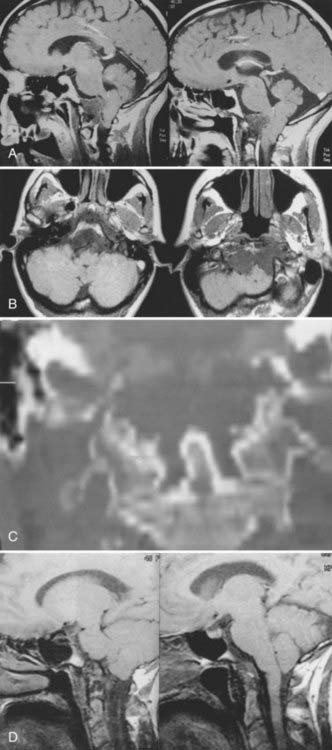
Cranial-based chordomas are clearly defined by the use of high-resolution CT, on which they appear as solitary or multiple areas of decreased attenuation within the clivus. T2-weighed MRI reveals a bright signal from the marrow in the skull base, signifying replacement of bone by tumor.9,25 At times, calcification is noted in the abnormal areas in the retropharyngeal space, representing sequestered bone fragments in tumor. Chordomas enhance with intravenous contrast and are well visualized by MRI. Chordomas are isointense on T1-weighted MRI (Fig. 308-2).
Presentation
Chordomas usually occur in adults, with a peak incidence occurring in the fourth decade of life.9,47,52 Less than 5% of these tumors arise in children, and they have a predilection for the spheno-occipital region.5 Headaches are often occipitocervical in location and aggravated by changes in craniovertebral positioning. In my series, 55% of patients benefited from craniovertebral stabilization in addition to tumor resection, owing to involvement of the occipital condyles.15,25 Lateral extension of these tumors can give rise to unilateral symptoms, such as hypoglossal nerve palsy. Larger tumors have the potential to cause both upper and lower cranial nerve palsies and a variety of problems related to brainstem compression. Chordomas often cause symptoms from local growth into the nasal cavity, pharynx, and paranasal sinuses.
Surgical Series
Forsyth and colleagues reviewed 51 intracranial chordomas treated surgically between 1960 and 1984 at the Mayo Clinic.52 The median age at presentation was 46 years, and 19 tumors were classified as chondroid chordomas. Eleven patients (22%) underwent biopsy, and 40 patients (78%) had subtotal resection. The survival rates for patients who underwent biopsy were 36% and 0% at 5 and 10 years, respectively, whereas survival rates for those with subtotal resections were 55% and 45% at 5 and 10 years, respectively. Patients who underwent postoperative radiation therapy tended to have longer disease-free survival times. Disease-free survival was the same for patients with chondroid chordomas as for those with typical chordomas.
Watkins and associates described 38 patients treated at the National Hospital of Neurology and Neurosurgery in London between 1958 and 1988.53 Craniotomies were used in 28 patients, and transoral or transmaxillary routes were used in 10 others. All patients underwent postoperative external-beam radiotherapy of 50 to 60 Gy. Recurrence developed in 23 patients, and 13 died within 5 years. Twelve patients were lost to follow-up. The authors concluded that two groups existed: one with indolent disease and another with aggressive growth and poor outcome.
In a more recent publication in 2001, Crockard and associates described a multidisciplinary approach to skull base chordomas.54 A primary first time surgery was done in 24 and redo surgery (initial surgery elsewhere) in 18. A total removal was made in 2, radical 30 and subtotal or partial in the remainder. However, morbidity was significant, with CSF leak in 15, meningitis in 3, new cranial nerve abnormalities in 6 and persistent dysphonia in 4 patients. Three patients died.
Gay and colleagues reviewed the management of 46 chordomas and 14 chondrosarcomas involving the cranial base between 1984 and 1993 at the University of Pittsburgh.47 They recommended an aggressive approach to achieve long-term recurrence-free survival. Fifty percent of patients had undergone previous surgery before referral, and 22% had undergone previous external-beam radiation therapy. The surgical approach was a subtemporal-infratemporal fossa approach, sometimes combined with a transpetrous approach. In other instances, an extended subfrontal approach was used, and in a few cases, the lateral transcondylar approach was used. There was a high tendency to stay between the subtemporal-infratemporal fossa approach and the extended subfrontal approach. Using this technique, the rate of total or near-total resection was 67%. Eighteen patients had died by the 5-year follow-up. Postoperatively, 20% of patients underwent external-beam, proton-beam, or gamma radiation therapy. In patients who had total resection, the overall 5-year recurrence-free survival rate was 84%, compared with 64% in those with partial resection. However, the rate of morbidity was high. Thirty percent developed CSF leaks, 10% experienced meningitis, and 80% had an immediate new cranial nerve deficit. Using the Karnofsky performance score, 40% of patients had permanent functional deterioration. Based on this experience, the authors advocated aggressive initial surgical resection, with the sparing application of radiation therapy.
In 1997, Al-Mefty and Borba reported their results with an aggressive surgical approach combined with postoperative proton-beam therapy in 25 patients treated between 1990 and 1996.9 Radical or subtotal (>90%) removal was achieved in 84% of patients undergoing multiple procedures, when necessary, and extensive drilling of bone beyond the limits of tumor involvement. Postoperatively, 68% of the patients received a mean of 68-cGy–equivalent proton-beam radiotherapy. The postoperative mortality rate was 4%. The postoperative rate of morbidity, however, was 48%, although only 8% suffered permanent neurological deficits. Eighteen percent of the patients treated with proton-beam therapy developed radiation necrosis. The mean follow-up was only 25 months, however, making conclusions about outcome and survival difficult.
In a subsequent follow-up, Colli reviewed the series of Al-Mefty published in 2001.51 There were 55 chordomas and 10 chondrosarcomas. Follow-up of 1 to 150 months was reported, with a median of 38 months. A radical or subtotal removal was performed in 77.8% of patients. At 5-year follow-up, the mortality rate was 14.3%. The recurrence-free survival rates were 100% for chondrosarcomas and 50.7% for chordomas. Of those who received proton-beam therapy, 90.9% survived, as opposed to only 19.4% who received conventional postoperative radiation therapy. However, the complication rate was 60%, and 28.6% had a permanent worsening in neurological deficit. A transient deficit was present in 22.8%, which included new cranial nerve palsy, CSF leak, and hydrocephalus. The authors concluded that the survival was best with radical resection followed by proton-beam therapy.
Maira and coworkers37 achieved total tumor removal in 7 of 10 patients undergoing repeated transsphenoidal procedures for chordoma. They reported no evidence of disease in these patients at a mean of 38 months after surgery and encountered a cranial nerve complication in only 1 patient.
Couldwell and colleagues reported on a variation of the standard transsphenoidal approach to the sella with emphasis on extended approaches and parasellar approaches.36 This series of 105 patients reported in 2004 included 18 clivus chordomas. Total removal was accomplished in 66%. A hemiparesis was seen in 1 patient and new cranial nerve deficits in 2 patients, and an internal carotid occlusion was also found. Three patients had internal carotid artery hemorrhage, and 1 patient had a CSF leak, which was persistent. No deaths were reported.
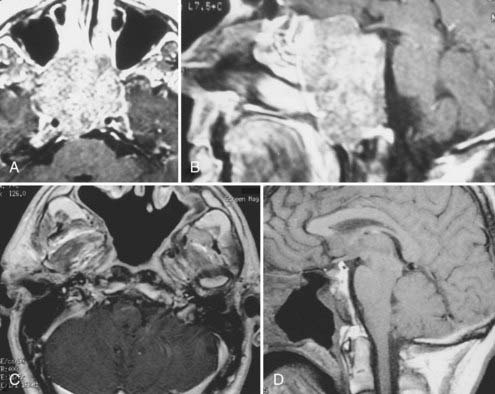
During the past 15 years, cranial-based exposures of the clivus and upper cervical-craniovertebral chordomas have become accepted. Thus, it is important to compare recent series with at least a 5- to 10-year follow-up. Since 1985, 25 males and 18 females with cranial-base chordomas have been treated at the University of Iowa Hospitals and Clinics.15,25 Fourteen (34%) had undergone previous surgery; 11 (25%) had undergone previous radiation therapy, 5 of whom had proton-beam therapy. The 43 patients underwent 49 skull base procedures, and 9 required stabilization. Fifteen patients underwent a transoral-transpalatopharyngeal approach, 8 underwent a transmaxillary approach, 6 underwent a transsphenoethmoidal approach, 4 underwent an infratemporal fossa primary approach, 4 underwent a lateral extrapharyngeal approach, 6 underwent a transcondylar approach, 5 underwent a transfacial approach, and 1 underwent a transbasal approach. Gross total resection was possible in 12 of the 43 individuals (Fig. 308-3). Subtotal resection (>90%), which was documented on postoperative MRI, was achieved in 14 individuals. Seven patients died during the 15-year follow-up period. All patients had typical chordomas and underwent detailed histologic and immunohistochemical analysis of the tumor. Of the 15 individuals who underwent a transpalatopharyngeal approach, 5 died, 3 patients within 2 years of the transoral procedure at my institution. Each of these 3 patients had previously undergone more than four operative approaches to the tumor and had also undergone proton-beam therapy. The time from proton-beam radiation to the recurrence presenting at my institution was less than 3 years. There were no cases of postoperative CSF leakage, meningitis, or new cranial nerve deficits.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree