– Eclampsia-associated vasoconstriction
– Drug-induced angiitis (“angiitis” is a misnomer as there is no vessel inflammation)
– Migraine “angiitis” (again, a misnomer)
– Thunderclap headache with reversible vasospasm
– CNS pseudovasculitis
– Benign angiopathy of the central nervous system (CNS)
– Call’s syndrome, or Call–Fleming syndrome
Transfemoral angiography or indirect computed tomography angiography (CTA) or magnetic resonance angiography (MRA) documenting multifocal segmental cerebral artery vasoconstriction No evidence for aneurysmal subarachnoid hemorrhage (SAH) Normal or near-normal cerebrospinal fluid (CSF) analysis (protein level <80 mg%, leukocytes <10 mm3, normal glucose level) Severe, acute headaches, with or without additional neurologic signs or symptoms Reversibility of angiographic abnormalities within 12 weeks after onset. If death occurs before the follow-up studies are completed, autopsy rules out such conditions as vasculitis, intracranial atherosclerosis, and aneurysmal SAH, which can also manifest with headache and stroke |
RCVS is more common in women than in men (approximately 2:1 to 4:1). It typically occurs between the ages of 20 and 60 years, though children can be affected. One-third of cases are considered idiopathic and may overlap with certain “primary” headaches including primary cough headache, benign sexual headache, and benign exertional headache. The other two-thirds are associated with hormonal influences (e.g., obstetric delivery), vasoactive substances (such as medications or blood products), or mechanical forces (such as surgery or trauma). Due to distinct yet overlapping clinical, laboratory, and imaging findings, primary angiitis of the CNS (PACNS) remains on the list of differential diagnoses. Imaging in RCVS typically shows reversible but prolonged multifocal segmental vasoconstriction, which probably progresses distally to proximally, and is predominantly intracranial. The time course of imaging changes does not always correlate with symptoms, such as thunderclap headaches or focal neurological signs [4]. Management can be a combination of symptomatic (pain and anxiety), supportive (blood pressure control), and disease-specific (calcium channel blockers) treatments (Table 13.3). Intravascular intervention may be indicated in more severe and clinically progressive cases, as progression to death has been documented in exceptional cases. Headaches may recur for a few weeks. Most patients recover completely although visual deficits and mild hemiparesis can persist in a minority. Relapses are rare. It seems logical to discontinue potentially contributing medications such as selective serotonin uptake inhibitors (SSRIs), triptans, ergot derivatives, and over-the-counter sympathomimetics used as decongestants or diet medications [5].
Supportive and symptomatic measures – Observation – Discontinue vasoconstrictive medications – Seizure prophylaxis (long-term antiepileptic drugs unnecessary) – Intravenous fluids, analgesics, laxatives – Pharmacological blood pressure control is controversial |
Specific pharmacotherapy – Calcium channel blockers (e.g., nimodipine or verapamil) – duration 4–8 weeks or until headaches subside – Intravenous magnesium |
Interventions – Balloon angioplasty or intra-arterial vasodilators (nicardipine, milrinone, prostacyclin) may be attempted if progressive clinical decline |
Idiopathic RCVS
Case 1. Post-coital headache (source: Calabrese et al. [3])
A 46-year-old man with a history of common migraine with vascular risk factors of hypertension and hyperlipidemia presented with severe post-coital thunderclap headache. There was a recurrence of the headache on day 3, this time with coexistent cortical blindness and mild left hemiparesis. An emergent computed tomography angiography (CTA) showed the presence of multifocal segmental stenosis affecting multiple vessels including the basilar, posterior cerebral artery (PCAs) and superior cerebellar arteries (SCAs) as well as middle cerebral arteries (MCAs) bilaterally. Diffusion-weighted magnetic resonance imaging (MRI) showed symmetrical bilateral occipital lobe and small cerebellar and right frontal infarcts (Figure 13.1). CSF examination and laboratory testing was inconsistent with vasculitis or subarachnoid hemorrhage (SAH). The patient was treated with analgesia and verapamil. His symptoms abated in the following 3 weeks and magnetic resonance angiography (MRA) documented the resolution of the vasoconstriction.
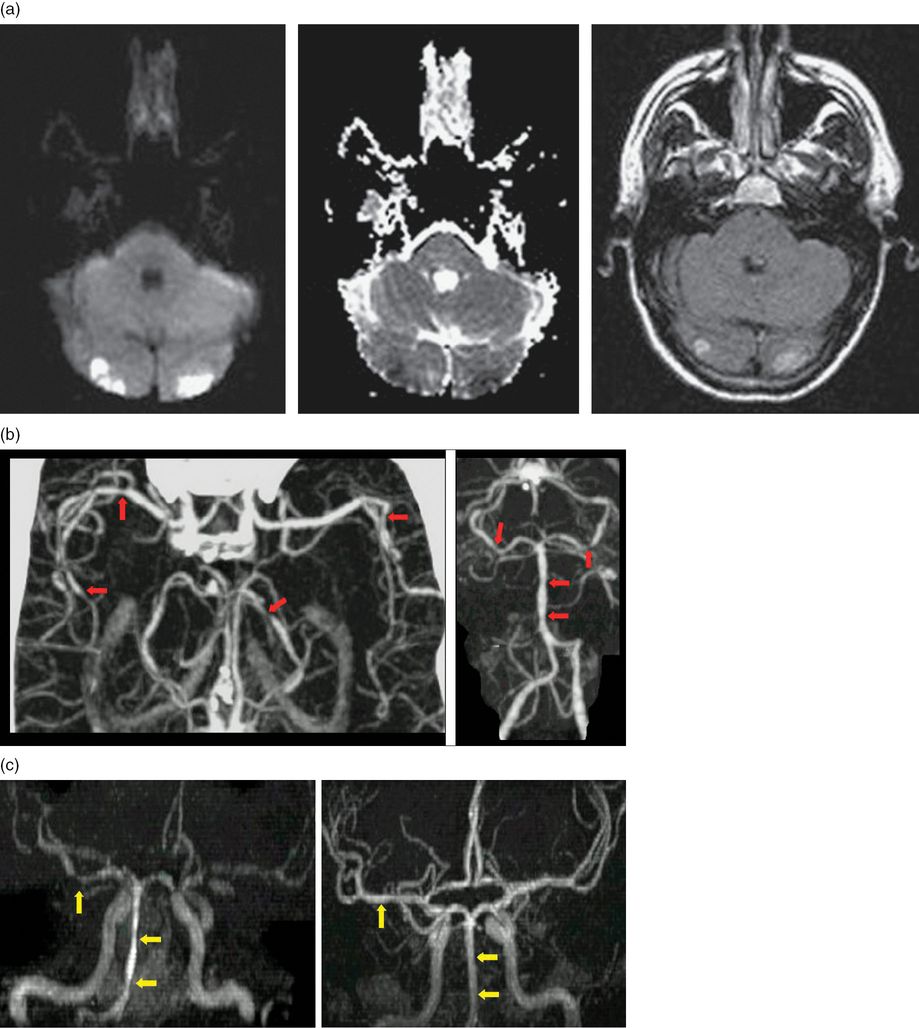
(a) Diffusion-weighted magnetic resonance imaging showing the presence of bilateral occipital infarcts. (b) CTA shows the presence of multifocal segmental stenosis in both MCAs bilaterally, the basilar artery, PCAs and SCAs. (c) The reversal of stenosis (the image on the right) compared to initial imaging (left). (Source: Calabrese et al., Ann Intern Med 2007; 146(1): 34–44.)
Discussion
Here we detail a patient who presented with thunderclap headaches which may have been diagnosed as primary post-coital headaches except for the recurrence of pain, followed by the development of cortical visual symptoms in the presence of widespread multifocal cerebral arterial narrowing. No hormonal, medication, or traumatic triggers were identified in this case. The patient did not have recurrences of RCVS during prolonged follow-up [6].
Primary and secondary headaches as mimics of RCVS
RCVS overlaps with a number of primary headaches including post-coital headaches, migraines, cough-induced headaches, and primary thunderclap headaches [4,7,8]. The distinction between these conditions and RCVS is the presence of prolonged reversible intracranial arterial narrowing. Whether the pathophysiologies of these idiopathic headaches are similar to RCVS or not requires further investigation.
Imaging in suspected RCVS
New onset thunderclap headaches merit urgent brain imaging to exclude several life-threatening conditions (Table 13.4)[9]. A recent small series suggests that the rate of RCVS in patients with thunderclap headaches (without evidence of hemorrhage) could be as high as 8.8% [10]. Imaging may demonstrate complications such as convexity SAH or brain edema (Table 13.5). Convexity SAH has a broad differential diagnosis (Table 13.6), but RCVS is the most common cause in young individuals below age 60 years, or in the setting of recurrent thunderclap headache. Magnetic resonance venography (MRV) or CT venography (CTV) is indicated when cerebral venous sinus thrombosis (CVST) is suspected.
With neck rigidity |
-Subarachnoid hemorrhage (SAH) |
-Meningitis, encephalitis |
Without neck rigidity |
-Cervical artery dissections |
-Acute ischemic stroke (PCA embolus) |
-Intracranial hematoma including pituitary apoplexy |
-Cerebral venous sinus thrombosis (CVST) |
-Intracranial hypotension syndrome |
-Hypertensive crises/pressor responses (e.g., pheochromocytoma) |
-Posterior reversible encephalopathy syndrome (PRES) |
-Reversible cerebral vasoconstriction syndrome (RCVS) |
-Sphenoid sinusitis (complicated sinusitis) |
–Primary thunderclap headaches |
Convexity (non-aneurysmal) SAH |
Brain edema |
Parenchymal hemorrhage |
Cerebral infarction |
Seizures |
Vascular anomalies |
Pial arteriovenous malformation Dural arteriovenous fistula Cavernous malformations Infectious (mycotic) aneurysms Cervical arterial dissections |
Obstructive and hemodynamic causes |
Dural and cortical venous sinus thrombosis Moyamoya Severe atherosclerotic carotid artery disease Posterior reversible encephalopathy syndrome (PRES) |
Increased risk of bleeding |
Coagulopathy Cerebral amyloid angiopathy (CAA) |
Destructive lesions |
Tumors |
Inflammatory causes |
Infective endocarditis |
Reversible cerebral vasoconstriction syndrome |
It is important to note that initial brain parenchymal as well as vascular imaging may be normal in patients with RCVS. If the index of suspicion is high, imaging should be repeated in a few days. Common findings include the presence of intracranial blood including intracranial hemorrhage, subarachnoid blood, brain edema, watershed infarcts, and multiple areas of vascular narrowing (beading).
RCVS in the obstetric population
Case 2. Late prepartum
A 27-year-old right-handed G1P0 woman in her 35th week of pregnancy was referred to the neurology service for acute severe headache and transient neurologic symptoms. She had been hospitalized for a high-risk pregnancy due to the presence of gestational diabetes and “mild” preeclampsia. During a prenatal clinical visit the patient was found to have raised blood pressure and proteinuria. Her hepatic, renal, and hematological status were stable. The patient was admitted to be monitored on the obstetrics ward as the date of delivery approached. She was treated with intravenous hydralazine and magnesium sulfate. She was also started on steroids to accelerate fetal lung maturity. During the hospitalization the patient had an acute onset of left face numbness which over the next 5 minutes spread to her left hand. The staff also noted the presence of mild slurring of speech. She complained of severe occipital headache. She had no visual symptoms: no scintillation, no blurring of vision, diplopia, or transient visual loss.
On examination, the patient’s blood pressure was 160/101 mmHg. General examination was normal except for bilateral pitting edema. She had no neck stiffness. Neurologic examination failed to show the presence of objective hypoesthesia or cerebellar signs. There was no hyperreflexia. A non-contrasted cerebral CT showed the presence of a small non-traumatic SAH in the right hemispheric high convexity (Figure 13.2a). The patient was transferred for an urgent CTA study, after her obstetric team deemed the risks acceptable in the third trimester, which confirmed the presence of the subarachnoid blood and additionally revealed the widespread presence of segmental intracranial vasoconstriction worse in the right anterior circulation (Figure 13.2d).
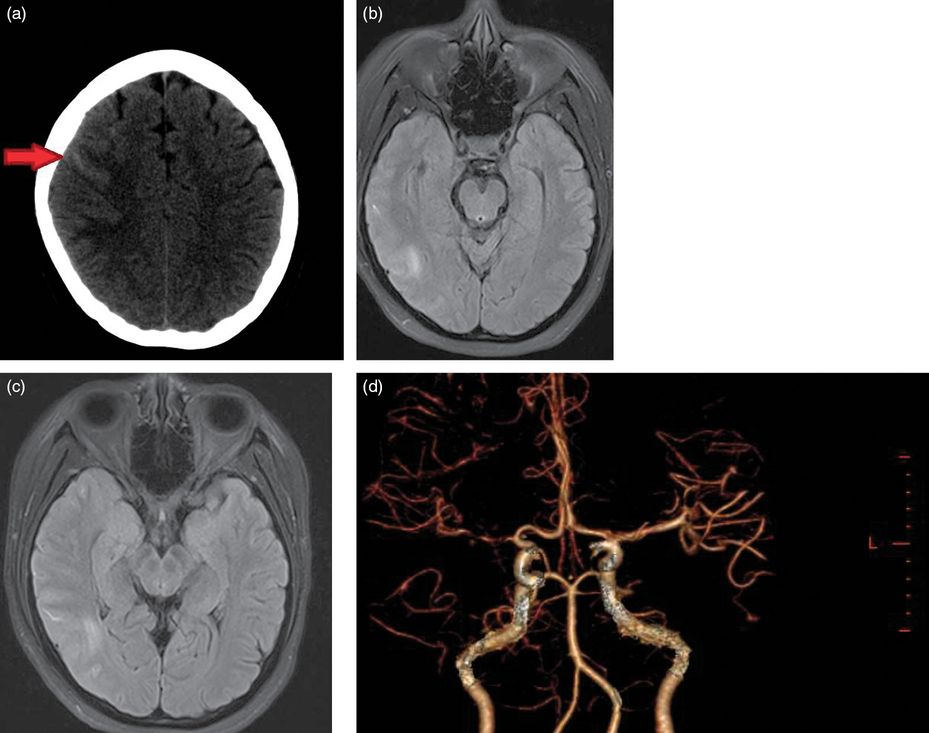
(a) Non-contrast brain CT with the arrow pointing to the presence of blood in the right hemispheric convexity. (b and c) Two cross-sections of FLAIR imaging which confirm the presence of blood and additionally show some subcortical edema. (d) A three-dimensional image reconstructed from CT angiography data which shows widespread areas of alternating narrowing and dilatation.
The patient was transferred to the high dependency unit for blood pressure monitoring. An extra loading dose of magnesium sulfate was given intravenously under the supervision of her obstetrician and oral nimodipine was instituted. Anxiolytics and pain relief were administered. An MRI was performed after she was stabilized. It showed a subjacent area of FLAIR signal hyperintensities consistent with vasogenic edema. Extensive work-up for vasculitis was found to be negative. She was transferred for a cesarean section as soon as she was stable and gave birth to a healthy child. She was kept in for observation for 10 days postpartum by which time her headaches had abated. Serial transcranial Doppler (TCD) documented the resolution of “vasospasm” over the period of her hospitalization.
Case 3. Early postpartum
A 34-year-old woman, G5P3, 1 week postpartum presented acutely with severe head pain with concomitant photophobia and abdominal pain. Her symptoms started acutely at 1 a.m. when she was awoken from her sleep with the worst headache of her life. She ignored the symptoms and the headache temporarily abated with time but recurred the afternoon of the following day. She went to a local hospital in the afternoon where she had an emergent CT which showed the presence of right-frontal intraparenchymal hemorrhage and bilateral frontal SAH with blood along the falx cerebri. She was taken emergently to the angiography suite where a catheter angiogram showed significant reduction in blood flow into the right middle cerebral artery secondary to severe M1 vasospasm. The patient was treated with intra-arterial calcium channel blocker which caused a reversal of the intracranial vasoconstriction (Figures 13.3a, b, and c). She was transferred to the intensive care unit (ICU) for close monitoring.
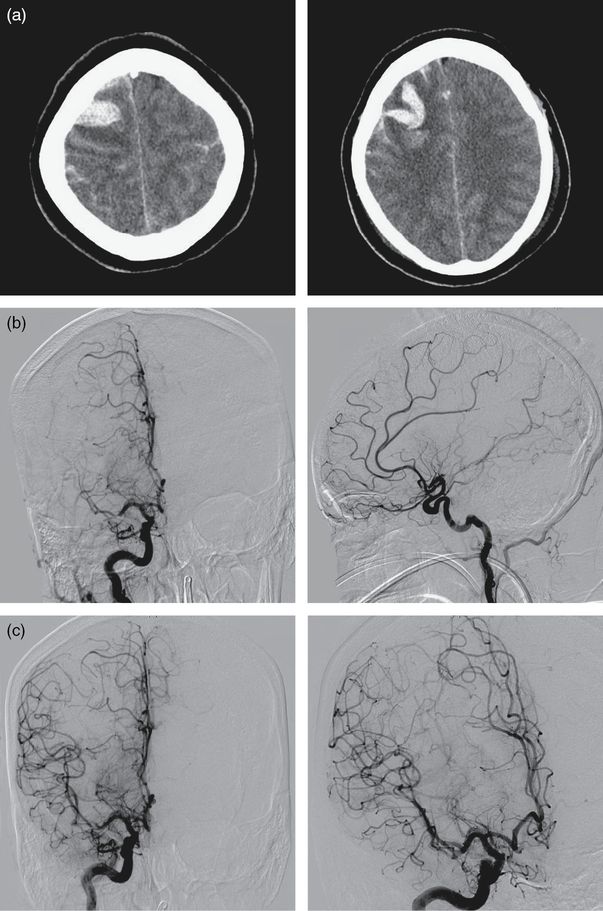
(a) Unenhanced head CT shows the presence of intraparenchymal and subarachnoid blood. (b) Catheter angiogram shows the presence of severe M1 vasoconstriction. (c) The same patient after intra-arterial calcium channel blockers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








