Chapter 59 Use of Clinical Tools and Tests in Sleep Medicine
Abstract
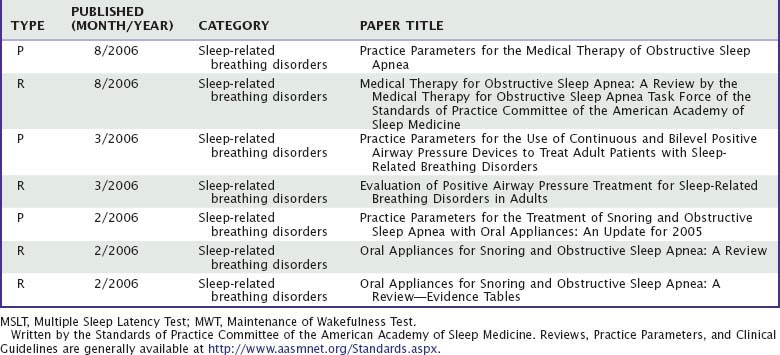
This chapter focuses on the comparative value of different approaches to clinical assessment of sleep-related problems. The symptoms, signs, and test results relevant to particular sleep disorders are described in detail in other parts of this volume. This chapter, instead, highlights the clinical reasoning process by which a clinician challenged with a sleep complaint can combine information from different sources, appropriately weigh available evidence, and arrive at sound diagnoses and treatment plans. Data are reviewed on the value of test results in evaluations of four common clinical problems: suspected obstructive sleep-disordered breathing, hypersomnolence, insomnia, and parasomnias. The Standards of Practice Committee of the American Academy of Sleep Medicine reviews the value of clinical tests and publishes guidelines. A selection of evidence-based practice parameters and reviews can be accessed at http://www.aasmnet.org/Standards.aspx (Table 59-1). These are recommended to supplement overviews presented in this chapter.
Table 59-1 Best Practice Guides (BPG), Clinical Guidelines (CG), Practice Parameters (P), and Reviews (R)
Evaluation for Sleep-Related Breathing Disorders
History and Questionnaires
Sleep-related breathing disorders are by far the most common class of disorders diagnosed at U.S. sleep centers, and obstructive sleep apnea (OSA) alone accounts for nearly 70% of all patients evaluated.1 The value of interview and questionnaire information has been studied with respect to polysomnographically confirmed diagnoses of OSA, a term now used to include the upper airway resistance syndrome, for which limited prior information also exists. For primary snoring, no objective measure has been shown to be better than subjective reports. Virtually no data are available on the utility of interviews and questionnaires in the diagnosis of nonobstructive forms of sleep-related breathing disorders.
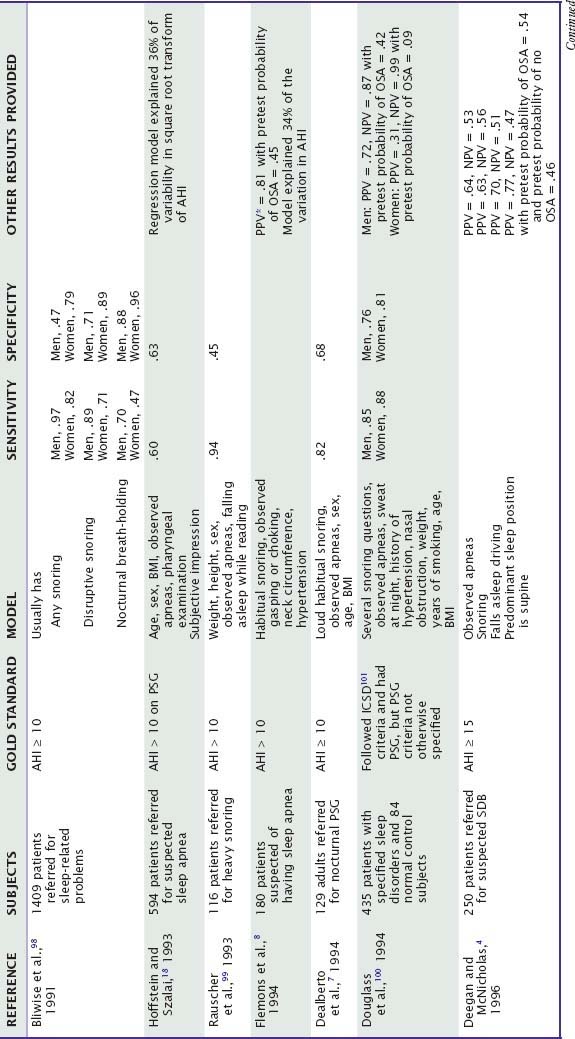
Subjective clinical impressions of OSA tend to have inadequate sensitivity (probability of a positive test result or assessment given that the disorder is present) and specificity (probability of a negative test result given that the disorder is absent). Combinations of some symptoms can have sensitivity above 0.90, but specificity is usually poor (Table 59-2). Performance of these models in clinical practice can be worse than originally reported.2 Most studies do not report the positive predictive value (PPV, probability that the disorder is present given a positive test result) and negative predictive value (NPV, probability that the disorder is absent given a negative test result). However, sensitivity and specificity data suggest that the NPV of some symptom combinations may be good, whereas the PPV is probably poor, especially when the prevalence of OSA in the tested population is not high.3 Accordingly, patients without a history suggestive of OSA usually do not receive further testing for it. Among patients referred for suspected OSA, models based on historical information may accurately classify a minority as apnea free without further tests.4 In practice, patients who do have symptoms of OSA generally are tested.
Table 59-2 Value of Historical or Questionnaire-Derived Diagnostic Information in the Diagnosis of Obstructive Sleep Apnea at Sleep Centers: Selected Studies Since 1990
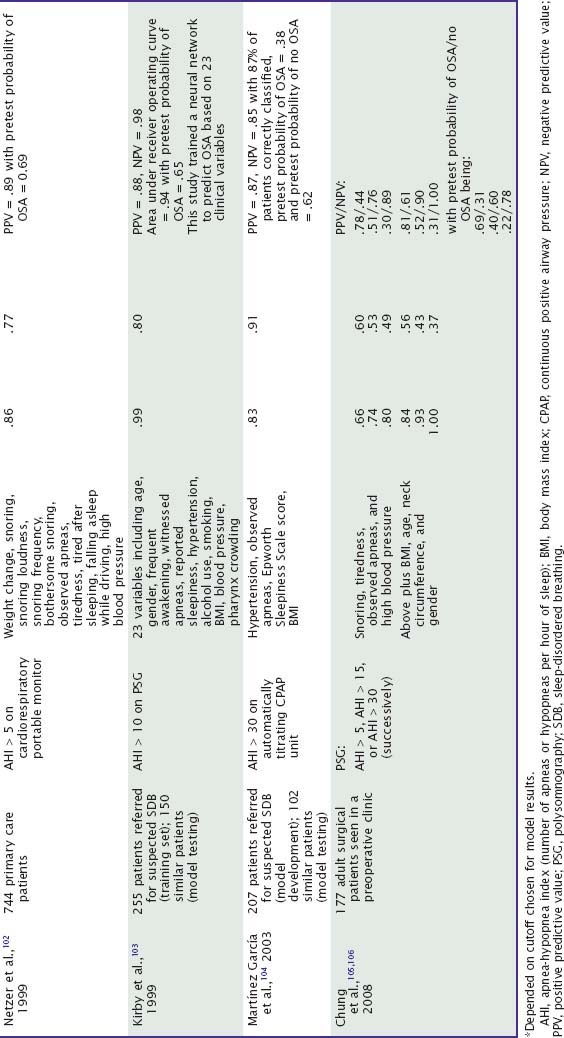
Although, in general, the PPV of symptoms alone is not high, a minority of patients have a clinical presentation so convincing as to be essentially diagnostic. However, the International Classification of Sleep Disorders requires minimal polysomnographic criteria, in addition to symptoms, to establish a diagnosis of OSA.5 Tests in such cases may also serve to define the severity of OSA. In a patient with a history strongly suggestive of OSA, the diagnosis must still be suspected when it is not confirmed by a single polysomnogram (which is not infallible) and especially when it is not confirmed by a more abbreviated home sleep study.6
The diagnostic values of specific symptoms are difficult to judge on the basis of studies with significant methodological differences. The author’s clinical impressions are listed in Table 59-3, with reference to adults referred for sleep evaluations. These data are not quantitative, but they illustrate how PPV and NPV for the same symptom may differ substantially. Clinical value may be quite different in other populations. For example, among patients referred specifically for possible OSA, the symptom of excessive daytime sleepiness may7 or may not8 be useful in making the diagnosis, and a history of hypertension may be better than a report of snoring as an indication that OSA is present.9 In the community, the symptom with the highest predictive value for OSA is habitual snoring, although excessive daytime sleepiness and observed apneas are also useful.10
Table 59-3 Estimated Clinical Values of Specific Symptoms in the Diagnosis of Obstructive Sleep Apnea Syndrome, with Reference to Patients Referred to a Sleep Clinic
| Effect on Probability of OSA | ||
|---|---|---|
| SYMPTOM | SYMPTOM PRESENT | SYMPTOM ABSENT |
| Habitual snoring | ↑ ↑ | ↓ ↓ ↓ |
| Loud snoring | ↑ ↑ ↑ | ↓ ↓ |
| Observed apneas | ↑ ↑ ↑ | ↓ |
| Sleepy while driving | ↑ ↑ | ↓ |
| Sleepy while reading | ↑ | ↓ ↓ |
| Morning headaches | ↑ | ↓ |
| Dry mouth on awakening | ↑ ↑ | ↓ |
| Nocturia (>1 episode/night) | ↑ ↑ | ↓ |
| Excessive sweating at night | ↑ ↑ | ↓ |
| Nocturnal reflux | ↑ ↑ | ↓ |
| Sleep maintenance insomnia | ↑ ↑ | ↓ ↓ |
| Nocturnal restlessness | ↑ | ↓ ↓ |
| History of hypertension | ↑ ↑ | ↓ |
| History of stroke | ↑ ↑ | ↓ |
↑ ↑ ↑, large increase; ↑ ↑, moderate increase; ↑, little or no increase.
↓ ↓ ↓, large decrease; ↓ ↓, moderate decrease; ↓, little or no decrease.
Little is known about the ability of historical symptoms to identify upper airway resistance syndrome and to distinguish it from OSA, and data to support the distinction appear not to have warranted separation of these diagnoses in the most recent nosology.5 The probability of upper airway resistance syndrome rather than more severe OSA may be increased by youth, female sex, functional somatic complaints, resting hypotension, and normal neck circumference. Patients with upper airway resistance syndrome appear more likely than those with more severe forms of OSA to have sleep-onset insomnia.11 The probability of upper airway resistance syndrome rather than primary snoring is increased by the presence of excessive daytime sleepiness or insomnia and may be increased by a history of hypertension, hypotension, dysmenorrhea, chronic fatigue syndrome, or bruxism.12–14 The likelihood of upper airway resistance syndrome rather than no sleep-disordered breathing is increased by a history of snoring, but the absence of snoring may not diminish the probability of upper airway resistance syndrome as much as it does the probability of more typical OSA.13
Physical Examination
Among variables related to body weight, neck circumference and body mass index (equal to weight in kilograms/height in meters squared) correlate well with the presence and severity of OSA in the community.15 Among patients referred for possible OSA, these variables still may be useful, but their predictive value is not large except in extreme ranges.4,7,8,16–18 Patients with OSA who are not obese often have pharyngeal crowding, obstructed nasal passages, or other craniofacial abnormalities associated with narrowing of the upper airway.19 The predictive value of such findings may differ somewhat between men and women.20 The Mallampati score, which reflects oropharyngeal crowding on a 4-point scale, was found in a series of 137 adults referred for OSA to predict polysomnographic confirmation of OSA.21 Each 1-point increase in the score was associated with an odds ratio of 2.5 (95% CI [1.2, 5.0]) for OSA and predicted a 5-point higher apnea-hypopnea index (coefficient = 5.3 [0.2-10]), independent of many other physical findings and symptoms.
Physical findings can also be combined into predictive quantitative models to aid in the diagnosis of OSA. One such model is based on body mass index, neck circumference, and bedside craniofacial measurements that include palatal height, maxillary intermolar distance, mandibular intermolar distance, and overjet.22 Among 300 sleep center patients, the model showed a sensitivity of 98% and a specificity of 100% for OSA. The PPV was 100% and the NPV was 89%. A second model is based on a modified Mallampati grade, tonsil size, and body mass index.23 This model showed a PPV of 90% and an NPV of 67%. A third model is based on simple neck profile, pharyngeal, and overbite scores; OSA was identified with 40% sensitivity and 96% specificity, which produced a PPV of 95% and an NPV of 49%.24 The neck profile measurement alone accurately excluded OSA in about one quarter of referred patients. Direct comparison of these three models is difficult because the studies used different recording techniques and criteria for OSA.
Some physical findings other than craniofacial features and obesity also have value in the diagnosis of OSA. High blood pressure increases the chance that OSA will be present, especially among those persons who are less obese.25 Signs of neuropathy or neuromuscular disease also may increase the likelihood of OSA.
Nocturnal Polysomnography
A nocturnal, laboratory-based polysomnogram (PSG) is the most commonly used test in the diagnosis of OSA. The PSG often is considered a gold standard for OSA diagnosis, assessment of severity, and identification of some other sleep disorders that can accompany OSA. The PSG allows direct monitoring and quantification of respiratory events and physiologic consequences—such as hypoxemia, arousals, and awakenings—that are believed to cause daytime symptoms. A single-night PSG is usually sufficient to diagnose or to exclude OSA. However, the test is not infallible. Accuracy may be reduced by variability in biologic severity, laboratory equipment, human scoring, or scoring protocols. Night-to-night variability may be particularly high in subjects with low but clinically significant rates of apneas and hypopneas during sleep. In one study, when 11 patients thought likely to have OSA on clinical grounds were restudied after initial normal PSGs, 6 had OSA.26
Although standards for scoring normal sleep were consistent and widely accepted for 4 decades,27 their widespread application to abnormal sleep probably did not serve patient care optimally well. Furthermore, standards for scoring sleep-related breathing abnormalities were not specified in the same document and became far from uniform between laboratories. Variations in practice made interpretation of unfamiliar reports difficult. Most important, the definition of hypopnea varied considerably between laboratories.28 The definition of apnea also varied to some extent.
Publication by the American Academy of Sleep Medicine in 2007 of new sleep scoring guidelines also included recommendations for polysomnographic equipment; scoring of abnormal respiratory events, electrocardiographic findings, movements, and arousals during sleep; and modifications necessary for children.29 These guidelines are required for sleep laboratories accredited by the Academy and thereby are serving to increase uniformity of procedures between laboratories in the United States. For sleep-disordered breathing, these guidelines also specify rules for optional scoring of respiratory event–related arousals (RERAs), defined by nasal or esophageal pressure monitoring, in the absence of apneas or hypopneas. Most laboratories report a summary measure, called the apnea-hypopnea index (AHI) or the respiratory disturbance index, that represents the sum total of apneas, hypopneas, and—sometimes in the second case—RERAs per hour of sleep. An index of this type is often given the most weight in interpretation of whether the study shows significant sleep-disordered breathing. Other commonly used results, the extent and frequency of oxygen desaturations, are also tallied and reported. Although the 2007 Manual has greatly advanced clinical care, it does have some limitations. The large majority of the recommendations were decided by consensus, in the absence of relevant published data, let alone outcome or evidence-based medicine. With respect to sleep-disordered breathing in particular, the hypopnea rule conforms to current Medicare billing requirements, but hypopneas leading to arousals or awakenings, in the absence of significant oxygen desaturation, are not recognized unless a rule labeled “alternative” is used. Furthermore, scoring of RERAs, by nasal or esophageal pressure monitoring, is “optional.” These multiple options, whether or not necessary given health care funding or widespread practice realities, leave significant disparities between laboratory practices, even among those accredited by the American Academy of Sleep Medicine. Furthermore, not all procedure details could be given in the Manual, and some are therefore left for individual laboratory directors to decide. As a result of this and also the imperfect reliability of PSGs, interpretation of PSG reports remains more complicated, especially when studies are performed by unfamiliar laboratories, and PSGs may not be definitive, particularly in borderline cases. Clinical information about the patient must still play an important role in establishing diagnoses and choosing appropriate management options.
Specifically, despite researchers’ tendency to use a specified AHI threshold to define OSA, the clinician cannot succumb to the same temptation. An individual patient with an AHI of 40 is at risk for excessive daytime sleepiness and cardiovascular morbidity but may not have either. Conversely, a patient with an AHI of 2 may still have OSA (e.g., because of night-to-night variability in test results) or may have upper airway resistance syndrome with associated sleepiness and morbidity.13,14 Interpretation of a PSG without knowledge of the patient’s clinical presentation can lead to serious underdiagnosis and overdiagnosis. Many clinicians believe that an AHI above 5 suggests OSA, but a large population-based epidemiologic study found that only 22.6% of women and 15.5% of men who met this criterion clearly complained of daytime hypersomnolence.15 Some patients who meet polysomnographic criteria for OSA but do not complain of hypersomnolence have little to gain from treatment.30
Further research is needed to define and to improve the ability of PSGs to measure those aspects of sleep-disordered breathing that most affect health and daytime sleepiness. The AHI and minimum oxygen saturation do not correlate strongly with daytime sleepiness,31 although the AHI may correlate better with cardiovascular morbidity.25 Some patients with RERAs have excessive daytime sleepiness in the absence of a significant AHI.13 Esophageal pressure monitoring, a gold standard in assessment of respiratory effort, facilitates identification of such patients and is particularly helpful when sleep-disordered breathing is evaluated in patients who lack obvious symptoms and signs.12,32 However, criteria for abnormal esophageal pressure recordings, as defined by association with poor outcomes, remain to be studied more definitively. Nasal pressure monitoring may provide a well-tolerated alternative, but identification of more apneic events by this sensitive measure of airflow is not necessarily a diagnostic advantage; studies have yet to demonstrate improved prediction of outcomes, and initial comparisons to thermistor results show correlations high enough (e.g., 0.90 or higher33) to suggest redundancy of information. Other polysomnographic measures that may (or may not) prove to enhance the ability of PSGs to predict outcomes of sleep-disordered breathing include end-tidal or transcutaneous carbon dioxide monitoring,34 pulse transit time,35 peripheral arterial tonometry,36 scoring of arousals,37 and analysis of respiratory cycle–related electroencephalographic changes.38
Modified Forms of the Polysomnogram
In comparison to the standard PSG, daytime nap studies and split-night studies may reduce costs and expedite evaluation. Studies of daytime PSGs have sometimes found a high NPV, with lower PPV, but inconsistent results and the lack of sufficient data explain why daytime PSGs have not generally been recommended.3 A successful split-night study may save a patient from a second night in the sleep laboratory. Initial studies of diagnostic accuracy and treatment outcomes appear promising.39 Although the preferred approach is separate, full night studies for diagnostic assessment and then positive airway pressure titration, split-night studies are now thought to be adequate alternatives in most cases.40
Portable Recording
A Portable Monitoring Task Force of the American Academy of Sleep Medicine recommended home studies only after a comprehensive sleep evaluation by a clinician board eligible or certified in sleep medicine, and then supervised and interpreted by someone with the same level of specialty training.6 This is because published literature about the effectiveness of home studies usually included screening clinical evaluations, differential diagnoses can be broad, home studies must be interpreted with sufficient knowledge of their many limitations in addition to capabilities, and results must be integrated into the context of the patient’s history and physical examination. Under these conditions, home studies can be used as an alternative to laboratory-based PSGs when the clinical judgment suggests that pretest probability of moderate to severe OSA is high. Home studies should generally not be used when the patient is a child or older person, significant health comorbidities (such as severe pulmonary disease, neuromuscular disease, or congestive heart failure) are present, additional sleep disorders besides OSA are suspected, or a screening process is needed for asymptomatic individuals, even if at risk because of comorbidities such as hypertension or obesity.6 Home studies may be indicated for patients who do not have access to laboratory-based PSG, cannot tolerate the procedure, or need follow-up assessment of response to non–positive airway pressure treatments of OSA.
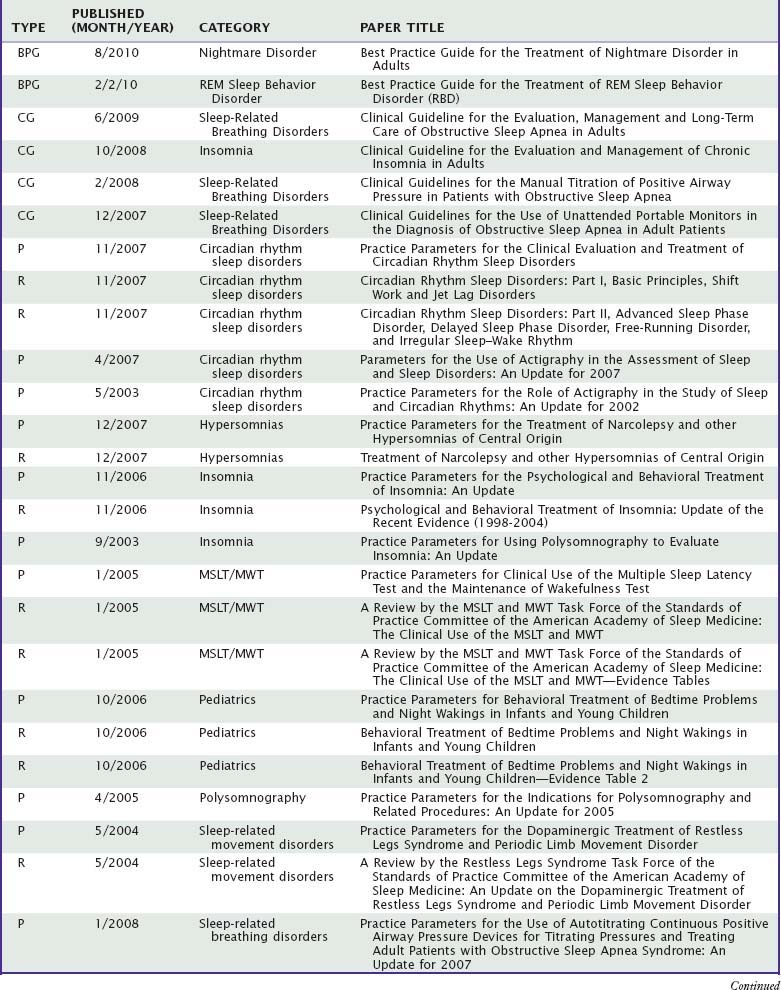
A recent randomized but not blinded clinical trial supported use of home oximetry as an alternative to more elaborate studies, but only in combination with a sleep specialist evaluation, threshold scores on several screening surveys, absence of significant comorbidities, and other circumstances that in combination applied to less than 4% of referred patients.41 Published guidelines for home studies recommend that at minimum they monitor airflow, respiratory effort, and blood oxygenation, and the equipment should be applied by a sleep technologist or health care practitioner with appropriate training.6 A normal home study generally must be confirmed by a more definitive laboratory-based sleep study. A suggested algorithm for use of home studies is shown in Figure 59-1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree