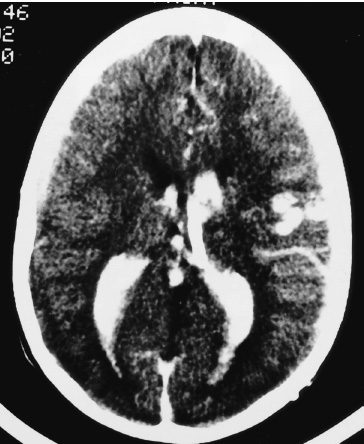
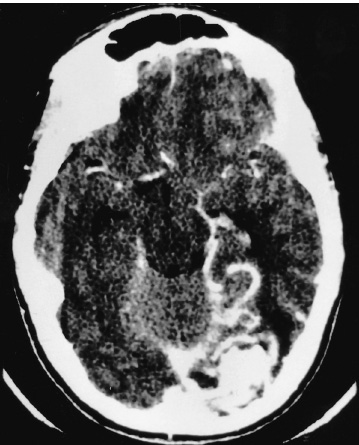
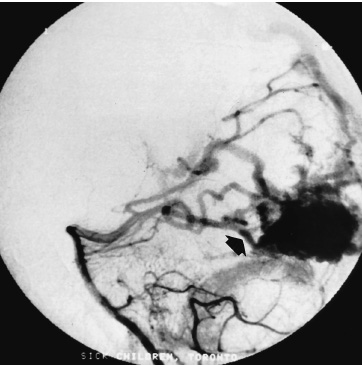
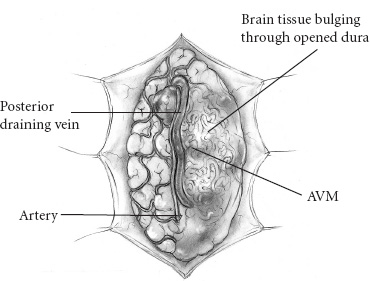
Vascular Disease The presenting symptom of eighty percent of arteriove-nous malformations (AVMs) in children is spontaneous intracranial hemorrhage. The hemorrhagic stroke in children, in particular, often has a devastating presentation that demands early intervention. It is now much easier to define the primary cause of the brain hemorrhage and to map a treatment plan that is best suited for it and its attendant phenomena. The operative evacuation of a cerebral clot is no longer the only strategy for a child’s impaired neurologic features. Clot removal may be one component of the therapy, but today’s technology has resulted in more effective treatments for AVMs in children. Because the operation for an AVM can be tedious and protracted, it is desirable to plan the procedure as one that is elective. Thus, if only subarachnoid or intraventricular hemorrhage has occurred or if the intracerebral hemorrhage is small and the patient’s condition is stable, interval surgery may be staged after a few days’ wait. Sometimes when the patient’s condition remains unchanged or even improved slightly, it is tempting to delay the operative intervention further, perhaps with a view to examine other, nonsurgical therapies; however, the startling incidents of hemorrhage as the presenting symptom for a child’s AVM and the likelihood of repeat hemorrhage as well as the remarkable recoveries that the child may demonstrate immediately after the hematoma is removed all validate an operative solution for this type of stroke problem. Successful AVM management is dependent on the AVM’s location, size, hemodynamics; the patient’s clinical condition; and the treatment method selected. The treatment goals should be to preserve life and neurologic function while achieving complete removal of the AVM and preserving the normal cerebral circulation. A certain number of children, without warning, will experience the onset of a severe, generalized headache that is not representative of any organic pathology within the cranium. Computed tomography (CT) imaging usually sorts out these cases at the primary, referral level. When the CT scan demonstrates a cerebral hemorrhage from a child’s ruptured AVM, blood clot and perhaps calcification are seen; and on enhanced study, the dilated feeding and draining vascular channels and large blood-containing varices may be seen (Figs. 20–1 and 20–2). Magnetic resonance imaging (MRI) is capable of defining the nature of the hemorrhage as well as the involved vasculature. In reality, however, the urgent nature of the child’s condition often mitigates against the consumption of time necessary for a properly performed MRI. Additionally, the surgeon should not rely on the vascular anatomy as revealed on the MRI to inform one fully of its abnormalities. Most AVMs that bleed are small, especially in children. The tiny feeding and draining vessels associated with a nidus of varying size may be completely obscured on MRI, particularly when there is blood clot present in addition. The MRI is a most valuable adjunct in the elective situation, especially for small lesions adjacent to the ventricular wall or within the diencephalon or brainstem. The valuable information obtained from this imaging technique could change the type of therapy. FIGURE 20–2. This left occipital arteriovenous malformation proved to be fed by a large branch from the posterior cerebral artery. Selective carotid and vertebral arteriography, with superselective views if necessary, remains the standard of investigation in a child with an AVM. The numbers and location of the arterial feeders can be examined by films processed with subtraction and magnified techniques (Fig. 20–3). Seldom is a malformation served only by one arterial channel. There may be only one major artery contributing to the lesion, but the surgeon should anticipate any number of minor vessels coming from pial or deep perforating sources, especially in relation to the underlying ventricle. On the other hand, the major venous drainage from an AVM in a child is frequently through a solitary large cortical vein, or one ending in the deep venous system. This venous outflow is usually precisely defined on arteriography. FIGURE 20–3. The direct arterial supply to the occipital arteriovenous malformation is demonstrated (arrow). Cerebral vasospasm is seldom a problem in children with AVMs, but rebleeding during the waiting interval may be. Thus, antispasm, antihypertensive pharmacologic measures usually are not required. If the clot is large, it is advisable to initiate an anticonvulsant drug because of the threat of delayed seizures. If the operation possibly can be delayed for 3 to 7 days after the hemorrhage, the surgeon will be rewarded with a properly prepared patient whose relaxed brain contains a partially liquefying hematoma. A certain percentage of patients will arrive in a precarious condition because of the size and location of their brain hematoma. The latter may be sufficiently large to obscure vessel abnormalities on CT scan. Under these circumstances, the surgeon has no option but to perform a craniotomy for clot removal. As the cause of the child’s cerebral hemorrhage is still unknown at this stage, the surgeon should confirm that it has not arisen from an undiagnosed coagulopathy. With alleviation of brain compression, the stage is properly set for an interval, elective arteriogram. The urgency is not the same for the 20% of patients whose presenting symptom is other than spontaneous hemorrhage. This group has been investigated in a traditional fashion for seizures, headaches, and perhaps developmental delay. The malformation has been discovered during the testing or even during elective surgery. The clinical challenge is to determine in advance whether surgical obliteration of the malformation will influence the seizure events, headache, or developmental problem and whether, therefore, it should be undertaken. With appropriate lines placed and controlled ventilator cycles, the patient has adequate brain relaxation for tissue exposure and dissection. Induced hypotension is not usually required for pediatric AVM surgery, nor is cerebral perfusion pressure breakthrough a frequent complication. Cerebrospinal fluid (CSF) evacuation through a lumbar drain to facilitate brain relaxation is often difficult to achieve, especially in the young child. Finally, the surgeon should decide with the anesthetist whether mannitol is to be administered during the craniotomy. The patient’s body position should be such that maximum comfort is ensured to all the major pressure points and extremity joints in the event that surgery lasts for several hours. This is especially the case with regard to positioning of the shoulders, arms, and cervical spine. The table is flexed appropriately to elevate the head slightly above the heart, and the headrest position should ensure that the area of brain to be explored is parallel to the floor. If stereotactic localization of the lesion is not to be used, the surgeon must rely on traditional surface landmarks of the skull (and thus the brain) to plan a generous flap. These take into account not only the three-dimensional size and location of the AVM but also the associated hematoma, especially if it extends away from the malformation. No one can be faulted for being overly generous with the scalp and bone flaps. If a localizing technique has been used to isolate a small malformation, especially one located in critical areas of the brain, then the overall exposure and bone flap can be reduced accordingly. Arriving at the dura, the surgeon may be somewhat concerned that it feels snug or is bulging. If the anesthetic management to this point is ideal, the surgeon should expect that there would be a degree of bulkiness to the brain, even when the hematoma volume is minimal. If peripheral dural tack-up sutures are part of the surgeon’s regular procedure, they should not be put in until the end of the procedure. Doing so beforehand runs the risk of impaling the brain or entering surface vessels of the malformation. It is unusual for a child’s AVM to obtain arterial supply from the external carotid system. Hence, there are few concerns when the dura mater is opened over the lesion, except around the region of the major draining vein. This structure is often so engorged that it becomes lightly adherent to the inner layer of dura, from which it can be separated by means of blunt dissection as the dura is reflected (Fig. 20–4). With the dura opened (and it should not be reflected more than necessary to visualize the malformation), the surgeon will notice that the brain “pouts” through the opening, indicative of its engorged stage. Unless there has been fresh bleeding, this minimal herniation usually resolves on its own. A variety of surface hallmarks may guide the surgeon to the site of the lesion. The most common is a generous arterialized draining vein that arises from a sulcus and heads to a major venous sinus. The most reliable surface markings are the veins because, regardless of their origin, at some point they all cross the brain’s surface (Fig. 20–5).

SURGICAL INDICATIONS AND PREOPERATIVE EVALUATION
PREOPERATIVE MANAGEMENT
Anesthetic Techniques and Positioning
The Dura Exposed
Intradural Strategies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


