Vascular Syndromes of the Forebrain, Brainstem, and Cerebellum
Arterial Blood Supply
The aortic arch gives rise to three major vessels: the brachiocephalic, the left common carotid, and the left subclavian arteries. The brachiocephalic in turn gives rise to the right subclavian and the right common carotid arteries. The two common carotid arteries run upward lateral to the trachea to approximately the level of the fourth cervical vertebra, where each bifurcates into the external and internal carotid arteries (Fig. 22.1). The two vertebral arteries arise from their respective subclavian arteries medial to the anterior scalene muscle and join to form the basilar artery. After originating (first segment) from the subclavian artery, the vertebral artery traverses the foramina transversaria from C6 to C2 (second segment), loops around the atlanto-occipital joint (third segment), and finally pierces the dura passing through the foramen magnum to enter the intracranial cavity (fourth segment) to join the other vertebral artery at the pontomedullary junction. The basilar artery has a relatively constant course, beginning at or slightly below the pontomedullary junction and stretching the length of the pons, tapering to its termination at the pons—midbrain junction where it bifurcates into its two terminal branches, the right and left posterior cerebral arteries (PCA), at the level of the interpeduncular cistern. The basilar artery gives off perforating arteries along its length.
After reaching the ventral surface of the brain, the two carotid arteries and the two vertebral arteries distribute blood in three distinct patterns: median and paramedian arteries, short circumferential arteries, and long circumferential arteries. Three major arteries irrigate the cerebrum: anterior, middle, and posterior cerebral arteries, and three major arteries also irrigate the cerebellum: the posterior inferior cerebellar artery (PICA), the anterior inferior cerebellar artery (AICA), and the superior cerebellar artery (SCA). Likewise, three groups of perforators supply the striatum and thalamus: medial striate arteries from the proximal segment of the anterior cerebral artery; lateral striate arteries from the stem of the middle cerebral artery; and posterior striate arteries from the posterior cerebral arteries. The blood supply of the brainstem, labyrinth, cochlea, cerebellum, subthalamus, portions of the thalamus, and temporo-occipital areas originates from the vertebral-basilar system. The carotid and vertebral-basilar artery systems join at the base of the brain to form the anastomotic circle of Willis [85,86].
The Internal Carotid Artery
The internal carotid arteries irrigate the rostral parts of the brain: the cerebral cortex, deep white matter, basal ganglia, and diencephalon. The internal carotid artery (ICA) may be divided into three main segments: cervical, petrosal, and intracranial. The cervical segment of the ICA has no branches. It ascends vertically in the neck, extending from the common carotid bifurcation to the base of the skull. It then enters the base of the skull through the carotid canal in the petrous portion of the temporal bone. The artery crosses the foramen lacerum and enters the cavernous sinus. The petrosal segment gives off a caroticotympanic branch (to the tympanic membrane) and a vidian branch (artery to the pterygoid canal). The intracranial segment begins distal to the petrous segment and proximal to the anterior clinoid process. Presellar and juxtasellar portions of this vessel are distinguished. The juxtasellar portion lies within the cavernous sinus in close proximity to the oculomotor, trochlear, and abducens nerves (CN III, IV, and VI), and the ophthalmic and maxillary divisions of the trigeminal nerve (CN V). Meningohypophyseal branches (tentorial artery of Bernasconi and Cassinari, dorsal meningeal artery, and inferior hypophyseal artery) arise from the presellar and juxtasellar portions to supply the adjacent meninges and posterior lobe of the hypophysis. The ICA then pierces the dura mater medial to the anterior clinoid process, where it becomes the supraclinoid. The ophthalmic artery, the first major branch of the ICA, arises at the level of the anterior clinoid process. This vessel runs forward initially intracranially, then traverses the optic canal en route to the orbit. The ophthalmic artery gives off orbital, extraorbital (ethmoidal branches to the dura of the cribriform plate and planum sphenoidal and anterior artery of the falx), and ocular branches; the most important of the ocular branches is the central retinal artery. Other ocular branches include the long and short posterior ciliary arteries and the anterior ciliary arteries. Rich anastomoses exist between the ophthalmic and the external carotid artery branches.
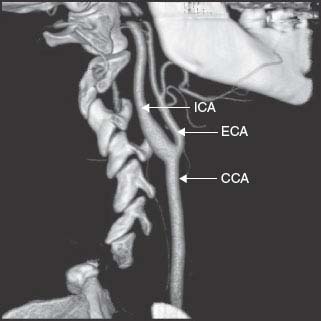
FIG. 22.1. Computed tomography angiogram of the carotid artery bifurcation.
After giving off the ophthalmic branch, the ICA gives rise to the posterior communicating artery and then to the anterior choroidal artery (AChA). The posterior communicating artery joins the posterior cerebral artery to form the posterolateral portion of the circle of Willis. The posterior communicating arteries may be large or threadlike and provide a link between the anterior and posterior circulations and between the two cerebral hemispheres. Penetrating branches from the posterior communicating artery supply the anterior and posterior hypothalamus, the optic tract and posterior portion of the optic chiasm, and the anterior and ventral thalamic nuclei. The AChA passes posterolaterally to reach the optic tract. When large, its territory includes the choroid plexus of the temporal horn, the hippocampus and dentate gyri, the amygdaloid nucleus, the piriform cortex and uncus of the temporal lobe, the lateral geniculate body, the optic tract and the origin of the optic radiations, the genu and the inferior and medial parts of the posterior limb of the internal capsule, the globus pallidus, the tail of the caudate nucleus, and the upper brainstem (middle one-third of the cerebral peduncle and substantia nigra).
After giving off the AChA, the ICA bifurcates to form the anterior cerebral and middle cerebral arteries.
The Anterior Choroidal Artery
The AChA vascularizes the posterior limb (posterior two-thirds) of the internal capsule, optic tract, lateral geniculate body (hilum and lateral part), optic radiation, amygdala, uncus and adjacent medial temporal lobe, and posterior paraventricular corona radiata.
The Anterior Cerebral Artery
The anterior cerebral artery (ACA) arises below the anterior perforated substance and runs anteromedially to the interhemispheric fissure, where it joins the opposite ACA by way of the anterior communicating artery, closing the rostral portion of the circle of Willis. The ACA supplies the medial surface of the cerebrum and the upper border of the frontal and parietal lobes [32]. It gives origin to (a) medial lenticulostriate branches, (b) pericallosal branches to the corpus callosum, and (c) hemispheric branches. The medial lenticulostriate branches include basal branches, which supply the dorsal aspect of the optic chiasm and the hypothalamus, and the medial striate artery (recurrent artery of Heubner), which supplies blood to the anteroinferior limb of the internal capsule, the anterior aspects of the putamen and caudate nuclei, and the tip of the outer segment of the globus pallidus. The callosal branches arise from the pericallosal artery, which is that portion of the ACA distal to the anterior communicating artery. Others reserve the term pericallosal artery for the segment beyond the origin of the callosomarginal artery. The ACA and the pericallosal arteries also supply the septum pellucidum and the fornix. The hemispheric branches supply the medial surface of the hemisphere and include the orbitofrontal, frontopolar, internal frontal (anterior, middle, and posterior), paracentral, and internal parietal (superior and inferior) branches.
The Middle Cerebral Artery
The middle cerebral artery (MCA), the largest branch of the ICA, arises below the medial part of the anterior perforated substance. It supplies most of the lateral surface of the cerebral hemisphere and the deep structures of the frontal and parietal lobes [26]. Three segments of the MCA are recognized: proximal, Sylvian, and distal. From the posterosuperior aspect of the proximal segment arise the penetrating lenticulostriate arteries, which nourish the adjacent corona radiata, external capsule, claustrum, putamen, part of the globus pallidus, body of the caudate nucleus, and superior portion of the anterior and posterior limbs of the internal capsule. Other branches that may arise from the horizontal segment are the orbitofrontal and anterior temporal arteries, but many variations occur. The Sylvian segment consists of all the branches on the insula of Reil and in the Sylvian fissure. The stem of the MCA divides, generally in one of three patterns: (a) bifurcation (78%), (b) trifurcation (12%), or (c) ramification into multiple trunks (10%). Therefore, shortly after the takeoff of the anterior temporal artery, the main trunk of the MCA most often bifurcates, one branch giving rise to the anterior or proximal group of arteries and the other branch to the posterior or distal group. The anterior group includes the orbitofrontal, precentral, central, and anterior parietal arteries. The posterior group includes the posterior parietal, posterior temporal, and the angular or terminal arteries.
The Posterior Cerebral Artery
The PCAs are the terminal branches of the basilar artery, although approximately 20%–25% of people have a fetal (embryonic) origin of the PCA. The PCA arises from the rostral end of the basilar artery within the interpeduncular cistern and supplies the occipital lobes and the inferomedial portions of the temporal lobes. Numerous other branches supply the mesencephalon, thalamus, and other structures. The branches of the PCA have been divided into three groups [126]: (a) the penetrating arteries to the brainstem, thalamus, and other deep structures, (b) the dorsal callosal artery, and (c) the cortical branches. From the origin of the PCA (as it surrounds the midbrain), numerous perforating branches are given off. Mesencephalic branches include the interpeduncular perforators and the short and long circumferential arteries. The arterial supply to the thalamus arises from the posterior communicating arteries and the perimesencephalic segment of the PCA. The dorsal callosal artery or splenial branch anastomoses with distal branches of the ACA. The PCA has four main cortical branches: the anterior temporal, posterior temporal, parieto-occipital, and calcarine arteries. The calcarine artery supplies the visual cortex.
Collateral Circulation
There are three main sources of collateral circulation to the brain that compensate in cases of carotid or basilar occlusion: (a) the circle of Willis, located on the ventral surface of the brain, that connects the internal carotid and vertebrobasilar arterial systems with each other, (b) anastomoses between branches of the extracranial and intracranial arteries, and (c) leptomeningeal anastomoses between the terminal branches of the major arteries of the cerebrum and cerebellum. The most important intracranial anastomoses are those of the circle of Willis. Atypical configurations of the circle of Willis resulting from hypoplasia of one or more component stems are found in 79% of individuals. Persistent primitive carotid basilar anastomoses may occur, such as (a) primitive trigeminal artery, (b) primitive acoustic (otic) artery, (c) primitive hypoglossal artery, and (d) primitive proatlantic artery. A persistent trigeminal artery [100] is the most frequent of the four primitive connections (0.1%–0.2% of adults) and may maintain significant collateral flow.
The arteries of the brain and their main territories of distribution are diagrammed in Figures 22.2 and 22.3.
Syndromes of the Cerebral Arteries
Cerebrovascular disorders result from either ischemia or hemorrhage within the central nervous system (CNS), and are broadly considered under the term stroke. A stroke is the third most common cause of mortality in most developed countries. A stroke indicates the relatively abrupt (seconds to hours) onset of a focal neurologic deficit resulting from disease (occlusion or rupture) of the arteries or veins that serve the CNS. Although stroke is commonly used to mean cerebral infarction (CI), it is preferable to use more precise terms, such as CI, intracerebral hemorrhage (ICH), or subarachnoid hemorrhage (SAH). The etiologic factors that may give rise to a stroke are many.
Other key factors are whether the deficit is transient or permanent, static or progressive, and whether the ischemia involves the cerebral cortex, subcortical areas, brainstem, or cerebellum. The neurologic deficit reflects the location and size of the lesion. Stroke syndromes may arise from an infarct or a hemorrhage. An infarct is usually due to either thrombosis from atherosclerotic lesions or embolism from the heart, aorta, or extracranial/intracranial vasculature. Hemorrhage may be epidural, subdural, subarachnoid, intra-parenchymal, or intraventricular, and may have various etiologies, including arterial hypertension, saccular aneurysms, arteriovenous malformations, blood dyscrasias, vasculitis, use of sympathomimetic drugs, cerebral amyloid angiopathy, trauma, or neoplasms.
Most cases of acute stroke are ischemic, usually resulting from thrombotic or embolic occlusion of a cerebral artery. Of all strokes, 87% are ischemic strokes. ICH is responsible for approximately 10% of all strokes and SAH accounts for the remainder. Cerebral atherothromboembolism involves predominantly the MCA, followed by the PCA territory; the ACA and the basilar artery are involved less frequently. In addition to extracranial atherosclerotic occlusive cerebrovascular disease, sources of cerebral embolism include atrial fibrillation, recent myocardial infarction with a mural thrombus, dilated cardiomyopathies, sick sinus syndrome, rheumatic valvular heart disease, prosthetic heart valves, congenital heart disease, cardiac tumors, and infective and non-bacterial thrombotic (marantic) endocarditis.
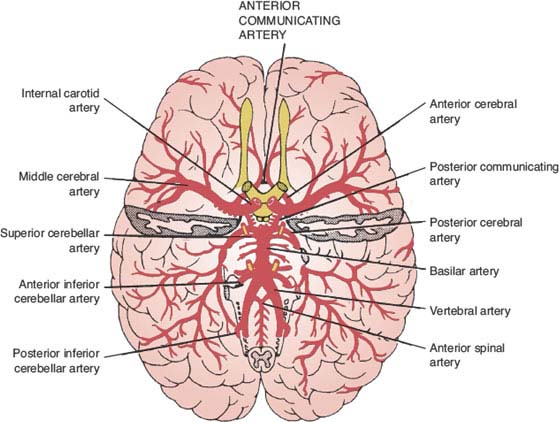
FIG. 22.2. The arteries of the brain (basilar view).
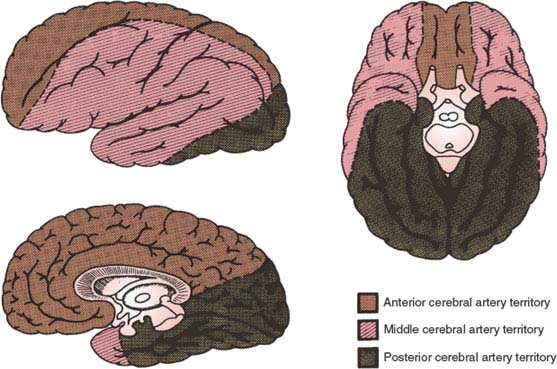
FIG. 22.3. Major territories of distribution of brain vessels.
Transient Ischemic Attacks
TIAs are powerful harbingers of stroke. Hemispheric TIAs have a greater risk for stroke and retinal TIAs. Approximately 10% of patients diagnosed as having a TIA have an ischemic stroke in the 90 days following the TIA diagnosis, with half of these having a stroke within 2 days of the TIA [149]. The ABCD2 score is useful for stroke risk stratification in patients with TIAs: ABCD2 scores of 4 or greater indicate a moderate to high stroke risk and justify prompt hospital admission. ABCD2 score: Age 60 or older = 1 point; Blood pressure ≥140/90 = 1 point; Clinical unilateral weakness = 2 points; speech impairment = 1 point; Duration 60 minutes or more = 2 points; less than 60 minutes = 1 point; Diabetes = 1 point [150]. TIAs are short-lived episodes of acute, focal, nonconvulsive neurologic dysfunction caused by reversible ischemia to the retina or brain. Onset of symptoms is sudden and often unprovoked, reaching maximum intensity almost immediately. TIAs commonly last 2 to 30 minutes. Patients often have no clinical manifestations by the time they present for medical attention [304]. The episode is followed by complete recovery. However, TIAs may be associated with variable rates of infarction on diffusion-weighted magnetic resonance imaging (DW-MRI) [20]. Thereby, a new “tissue based definition” of TIA has been proposed: brief episodes of neurological dysfunction caused by focal retinal or brain ischemia with symptoms typically lasting less than 60 minutes, and without evidence of acute infarction [4]. Because identification of the arterial territory involved is important in considering the extent of investigation and management, TIAs involving the carotid circulation are distinguished from those involving the vertebrobasilar circulation. TIAs are most often caused by thromboembolism associated with large vessel atherosclerosis, cardioembolism, or small vessel disease. Hemodynamic mechanisms are less common.
Approximately 25% of patients with TIAs complain of headaches during the attack. “Limb-shaking” TIAs may be associated with severe carotid artery stenosis [23].
Symptoms considered typical of TIAs in the carotid and vertebrobasilar circulation are shown in Table 22.1.
The Carotid Artery Syndrome
The only feature distinguishing the carotid artery syndrome from the MCA syndrome is amaurosis fugax or transient monocular blindness. Patients with amaurosis fugax often describe sudden onset of transient painless monocular loss of vision sometimes referred as a “curtain” or “shade” being pulled from the top or bottom of a visual field, or as a constriction of the visual field, such as an iris diaphragm type of monocular visual loss [139]. The former type of spell is most likely embolic, whereas the latter is most probably related to marginal perfusion causing diminished blood flow to the retina. Not infrequently, the characteristics of the attacks of visual loss are described as a temporary blackout, dimming, blurring, graying, or fogging of vision. Scintillations are seldom reported. In a subset of the North American Symptomatic Carotid Endarterectomy Trial (NASCET), one-third of patients reported an altitudinal visual loss with an ascending or descending shade [293]. Most attacks are spontaneous and unrelated to positional changes. Duration of visual loss is approximately 1 to 5 minutes; but rarely it may be 20 to 30 minutes. During attacks, the pupil is amaurotic and the retinal vessels collapse. Amaurosis fugax often results from embolism from the carotid artery, heart, or aorta, hypoperfusion, hypercoagulable states, or temporary angiospasm. Different types of microemboli can be seen in the retinal arterioles during or between attacks of transient monocular visual loss [336]. They are listed in the order of frequency in Table 22.2.
TABLE 22.1 Symptoms of Transient Ischemic Attacks
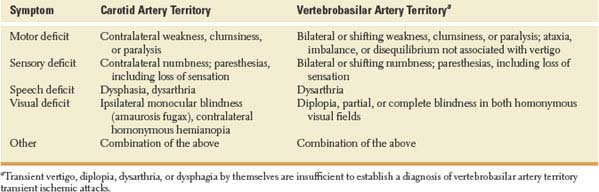
TABLE 22.2 Microemboli in Carotid Artery Syndrome

Unilateral loss of vision in bright light (“bright-light” amaurosis) may occur in patients with high-grade stenosis or occlusion of the ipsilateral carotid artery [111]. Episodic bilateral vision impairment related exclusively to light exposure might occur with bilateral high-grade stenosis or occlusion of the ICA [324]. Visual loss may persist for seconds to hours after exposure and is thought to be related to bilateral simultaneous retinal ischemia delaying regeneration of visual pigments in the pigment epithelial layer.
Differential diagnosis of transient monocular visual disturbances includes retinal ischemia (retinal migraine, vasospasm, Raynaud’s phenomenon, anemia, polycythemia, sickle cell disease, carotid artery compression or occlusion, postural hypotension, cardiac arrhythmia); optic disc elevation, dysplasia, or ischemia (intrapapillary drusen, optic nerve sheath meningioma, dysplastic coloboma, papilledema, giant cell arteritis, polyarteritis nodosa, eosinophilic vasculitis) [234,278]; and mechanical retinal or optic nerve stimulation (oculodigital phenomenon, lightning streaks of Moore, optic neuritis, retinal tear, flick phosphenes) [60,335]. Attacks of subacute angle closure glaucoma may also cause transient monocular visual loss [258]. Amaurosis fugax may also occur in association with the antiphospholipid antibody syndrome [55,91] and with exercise in healthy young adults (likely migraine equivalents) [143]. Patients with multiple sclerosis may also report uniocular or binocular dimming of vision after exercise (Uthoff symptom) [285]. Amaurosis fugax and ocular infarction in young adults and adolescents are associated with a more benign clinical course than those seen in older patients and are likely caused by migraine [301]. Rarely, an intraorbital tumor may compress the optic nerve or a nutrient vessel in certain gaze positions, causing transient monocular visual loss.
Atherothrombotic disease of the carotid system has a predilection for the bifurcation of the common carotid artery and proximal ICA. This is more frequent among whites and in men, whereas carotid artery siphon stenosis is more common among African Americans and Asians. Patients with carotid artery occlusive disease may present with recurrent TIAs, an apoplectic or stepwise onset, or a slowly progressive neurologic deficit. Atherothrombotic occlusion of the ICA is an important cause of ischemic stroke. Occlusion of the ICA in the neck may be asymptomatic in the presence of adequate collateral circulation, particularly if the occlusion develops slowly. Infarct patterns following ICA occlusion are heterogeneous [254]. Infarction of the homolateral hemisphere may occur when the collateral circulation is inadequate. Occlusion of the intracranial carotid artery bifurcation also called carotid terminus or carotid T occlusion typically results in large infarctions involving the MCA and anterior cerebral artery (ACA) territories, including deep structures perfused by the lenticulostriate arteries. In instances of a fetal origin of the posterior cerebral artery (PCA), the infarction will also involve the PCA territory. Decreased level of alertness and severe leg weakness are useful clinical clues to differentiate a carotid T occlusion from an MCA territory stroke.
Infarcts may involve the entire territory of the MCA (total), the areas of supply nearest the ICA or MCA (proximal), the border zone between the ACA and MCA (watershed), or only the white matter supplied by peripheral branches of the MCA (terminal). Patients may initially complain of headaches, and focal seizures may occur. Contralateral hemiplegia, hemianesthesia, homonymous hemianopia, and aphasia (if the dominant hemisphere is compromised) or apractagnosia (if the nondominant hemisphere is involved) may ensue. The association of amaurosis fugax or ischemic optic neuropathy with contralateral hemiplegia (optico-cerebral syndrome) is rarely seen [52]. Acute ICA occlusion may also rarely cause concurrent ophthalmoparesis (transient) with monocular blindness (permanent) [328]. Examination may show an ipsilateral Horner syndrome, usually transient, due to compromise of the sympathetic fibers coursing along the ICA. Ipsilateral optic atrophy seldom occurs. Ischemic oculopathy (ocular ischemic syndrome) can also be a manifestation of carotid artery occlusive disease [334]. Patients with ischemic oculopathy may complain of ocular or orbital pain often relieved by the supine position, decrease in vision, and “bright-light” amaurosis. There may be engorgement of conjunctival and episcleral vessels, corneal edema, ischemic pseudo-inflammatory uveitis, rubeosis iridis, and anterior chamber cells and flare [220]. The intraocular pressure may be low (early) or abnormally high (late). Occasionally, there may be asymmetric hypertensive retinal changes noted on funduscopy. Corneal arcus senilis may be less apparent on the side of low perfusion [286]. Venous stasis (hypotensive) retinopathy may occur with high-grade carotid stenosis or occlusion and is characterized by insidious onset, diminution or absence of venous pulsations, dilated and tortuous retinal veins, midperipheral retinal microaneurysms, blossom-shaped hemorrhages in the midperipheral retina, and retinal nerve fiber layer splinter hemorrhages. Hypotensive retinopathy may also include retinal arteriole narrowing, macular edema, and neovascularization in the posterior pole.
Typically, patients present with uniform (proportionate) hemiparesis (face, shoulder, hand, hip, and foot), or faciobrachial weakness. On rare occasions, small cortical infarcts may account for weakness limited to a particular group of digits, particularly the index finger [165,170], or finger extensors in cortical infarcts involving the contralateral middle to lower portion of the precentral gyrus [22,64,337]. Moreover, in rare instances, small cortical infarcts may cause pure sensory stroke [330], isolated dysarthria [174], asterixis [162], or upper limb monoataxia [237].
The neurovascular examination may disclose a well-localized bruit in the mid- or upper cervical area. Bruits arise when normal laminar flow of blood is disturbed, usually when the diameter stenosis is >50%. Cervical bruits may have many causes. The presence of a cervical bruit does not necessarily indicate underlying carotid atherosclerosis. Correlation with angiography or ultrasound studies show only approximately 60% agreement with cervical auscultation in predicting the presence of carotid stenosis, and may actually disappear with lesions causing diameter stenosis of >90%. Radiated cardiac murmurs, hyperdynamic states, nonatherosclerotic carotid arterial lesions (fibromuscular dysplasia, dissections, radiation vasculopathy), and venous hums can produce cervical murmurs. The absence of a bruit has little diagnostic value. The bruit may disappear when the stenosis is >90%. Conversely, a cervical bruit may be related to flow augmentation due to contralateral ICA occlusion.
Severe stenosis or occlusion of the ICA may cause progressive or episodic weakness of one lower extremity, often aggravated or precipitated by standing or walking [332]. This weakness is thought to be due to hypoperfusion in the border zone between the anterior and middle cerebral arteries. Also, episodic carotid ischemia may rarely cause intermittent limb shaking or repetitive involuntary movements [23,331]. These movements are brief, coarse, irregular or rhythmic, wavering or trembling, and affect the contralateral arm and hand, or arm, hand, and leg. They are characteristically precipitated by standing up, walking, or neck hyperextension and are promptly relieved by assuming the supine or sitting position and are thought to be due to transient hemodynamic ischemic episodes rather than epilepsy. Other atypical carotid distribution transient ischemic manifestations include orthostatic TIAs, transient anosognosia, and transient loss of pitch perception [99]. Infarcts of the genu of the internal capsule may cause contralateral facial and lingual paresis with dysarthria [48]. A cluster of TIAs (capsular warning syndrome) causing weakness of the contralateral hemibody and reflecting ischemia of a single lenticulostriate artery may occur hours to days before a stroke [94]. This capsular genu syndrome may also be associated with unilateral mastication-palatal-pharyngeal weakness, ipsilateral vocal cord paresis, and mild hand weakness (hand paresis suggests involvement of the anterior part of the posterior limb of the internal capsule). This faciolingual syndrome suggests that a majority of corticopontine and corticobulbar fibers to the facial and hypoglossal nuclei are located in the genu of the internal capsule; the absence of sternocleidomastoid paresis or sensory changes suggests that corticofugal fibers to the nucleus of CN XI and thalamocortical fibers corresponding to buccofacial sensation do not travel in the genu. The inconstant mastication, pharyngeal, palatal, and laryngeal weakness suggest bilateral, although predominantly unilateral, corticofugal projections to the motor nuclei of CN V and CN IX and the nucleus ambiguus or control of these functions by extracapsular fibers [48]. Faciolingual hemiparesis, whether associated with masseter, palatal, pharyngeal, laryngeal, or hand weakness, is highly suggestive of stroke limited to the genu of the contralateral internal capsule. Pure dysarthria, sometimes with contralateral facial weakness, may occur with striatocapsular infarction, with infarction of the superior portion of the anterior limb of the internal capsule or adjacent corona radiata, with infarction in the superior portion of the genu or adjacent corona radiata, with infarcts of the bulbar motor cortex, or with vertebrobasilar infarction [93,133,141].
Infarction involving the genu of the internal capsule has also been reported to result in behavioral changes [297]. Damage to the anterior nucleus of the thalamus, which lies immediately inferomedial to the genu of the internal capsule, cannot be ruled out in these cases. The acute syndrome includes fluctuating alertness, inattention, memory loss, apathy, abulia, and psychomotor retardation, suggesting frontal lobe dysfunction. Contralateral hemiparesis and dysarthria are mild, except when the infarct extends to involve the posterior capsular limb. Neuropsychological testing in patients with left-sided infarcts may reveal severe verbal memory loss, occasionally associated with dementia, whereas right-sided infarcts cause transient impairment in visuospatial memory. It has been inferred that the capsular genu infarct interrupts the inferior and anterior thalamic peduncles, resulting in functional deactivation of the ipsilateral frontal cortex (thalamocortical disconnection) [297]. Other authors have failed to see this syndrome with lesions in the genu of the internal capsule [48]. Trismus may seldom follow bilateral capsular genu infarctions [15].
The Anterior Choroidal Artery Syndrome
Infarction in the AChA territory typically results in hemiparesis due to involvement of the pyramidal tract in the posterior limb of the internal capsule, hemisensory loss for light touch and pinprick due to involvement of the superior thalamic radiations situated in the thalamolenticular portion of the posterior limb of the internal capsule, and homonymous hemianopia sparing the horizontal meridian or quadruple sectoranopia secondary to involvement of the optic tract, the lateral geniculate body, the optic radiations, or a combination of these [58,84,138]. A relative afferent pupillary defect may be present in the eye contralateral to the side of the lesion (optic tract lesion). Clinical syndromes with AChA infarction include a pure motor syndrome, a sensorimotor syndrome, and ataxic hemiparesis [138]. CT scan or MRI examination reveals abnormality in the posterior limb of the internal capsule, sparing the thalamus medially and encroaching on the tip of the globus pallidus laterally. A homonymous defect in the upper and lower visual fields sparing the horizontal meridian is characteristic of a lesion in the lateral geniculate body in the territory of the AChA [129]. In a small percentage of patients, AChA territory infarcts on the right side produce mild deficits of visual perception and visual memory for designs, left spatial hemineglect, constructional apraxia, anosognosia, and motor impersistence, and those on the left side produce a mild language disorder characterized by deficiencies with oral word association and dysarthria [46,84]. Bilateral AChA infarction may result in bilateral capsular infarction causing acute pseudobulbar mutism accompanied by varying degrees of facial diplegia, hemiparesis, hemisensory loss, lethargy, neglect, and affect changes [130]. Bilateral involvement of the lateral geniculate bodies may cause bilateral hourglass-shaped visual field defects. Fits of laughter or crying devoid of emotional content have also been described with AChA territory infarctions [88].
The Anterior Cerebral Artery Syndrome
Infarction in the ACA territory causes damage primarily to the medial frontal and parietal areas including the cingulate gyrus, basal aspects of the frontal lobes, rostrum, genu, trunk, and splenium of the corpus callosum, anterior portions of the diencephalon, head of the caudate nucleus, and anterior limb of the internal capsule.
Whether embolic, atherothrombotic, vasospastic, or the result of lacunar infarcts or arterial dissections, infarctions limited to the distribution of the ACA are rare, representing about 0.6% to 3% of CIs [12,47,153,274]. The clinical picture varies according to the site of occlusion and patency of available collaterals [47,79,263]. Infarction in the territory of the hemispheric branches of the ACA often results in contralateral weakness involving the lower extremity and, to a lesser extent, the arm (especially the shoulder). Patients may display lack of initiative or abulia. Paratonia (gegenhalten) and a grasp reflex may be present. With bilateral damage to the mesiofrontal region, patients may exhibit frontal lobe release signs, akinetic mutism, paraplegia, urinary incontinence, and amnesia with apathy [47,221]. With involvement of the anterior corpus callosum, they may have left arm apraxia (anterior disconnection syndrome) or hyperlexia [295]. Sensory examination may show contralateral tactile sensory loss affecting primarily the lower extremity. A number of patients have impaired articulation and a soft whispering voice. Bilateral ACA infarcts, unrelated to underlying anatomical abnormalities, occurred after viper envenomation [134]. With unilateral left-sided lesions, they may have transcortical motor aphasia. Some patients exhibit memory and emotional disturbances and impaired planning abilities. In some cases, there are disturbances of sphincter control with urinary incontinence (transient with unilateral lesions). Some patients demonstrate gait and postural disorders. Dominant medial frontal damage that includes the supplementary motor cortex may cause a disturbance of upper extremity control, including impaired bimanual coordination, the alien hand sign, and intermanual conflict [213]. Other manifestations include motor perseveration, ideomotor apraxia, amnesia, gaze deviation, anosognosia, and parkinsonism [153]. Large right ACA infarctions may cause a hemiplegia with the arm and leg affected more than the face, marked sensory neglect, impaired copying and micrographia [183].
Leg-predominant weakness with stroke is due to ACA infarction in only 25% of the cases. More often, it is related to lesions in the corona radiata or internal capsule, in the territory of the AChA or perforators (approximately 30%), or in the brainstem (approximately 25%) and can occur with lesions in the MCA territory or with thalamic hemorrhage [276]. Regarding lesions in the medial aspect of the frontal lobe, those restricted to the precentral gyrus portion of the paracentral lobule cause a contralateral, predominantly distal leg weakness. Lesions involving, in addition to the precentral gyrus, the premotor cortex and the supplementary motor area cause leg weakness, predominantly distal, and less severe proximal weakness of the arm. Lesions affecting the medial part of the premotor cortex and the supplementary motor area, while sparing the precentral gyrus, cause a contralateral hemiparesis, more pronounced in the leg, and predominating proximally in both leg and arm. Lesions of the internal capsule or brainstem cause proportional leg weakness. The weakness of the legs with these capsular and brainstem strokes suggests a somatotopic organization of the pyramidal tracts, with the leg fibers being probably dorsolaterally situated and the arm fibers situated ventromedially [276].
The syndrome of homolateral ataxia and crural paresis, with hemiparesis that predominates in the leg and homolateral ataxia in the arm, can occur with superficial ACA infarcts in the paracentral area [45]. Involvement of the corticopontocerebellar fibers at their origin along with damage to the lower limb motor strip or underlying white matter appear to cause this clinical syndrome in these cases. Ataxic hemiparesis has also been described with lesions of the pons, corona radiata, thalamus, lentiform nucleus, or other structures.
Infarction in the territory of the medial lenticulostriate artery (artery of Heubner) results in contralateral weakness of the face and arm without associated sensory loss. Therefore, with proximal ACA infarction, severe contralateral hemiplegia may result, with paralysis of the face, tongue, and arm from damage to the anterior limb of the interior capsule and paralysis of the leg from paracentral damage. Infarction of the basal branches of the ACA cause transient memory disorders, anxiety, and agitation. Patients with occlusion of the pericallosal branches may show apraxia, agraphia, and tactile anomia of the left hand. Distal occlusion of the ACA may cause infarction localized to the caudate nucleus (occasionally extending to involve the anterior limb of the internal capsule and anterior putamen), resulting in slight, transient hemiparesis, dysarthria, behavioral and cognitive deficits (e.g., agitation, hyperactivity, abulia, contralateral neglect), and language impairment [63].
Movement disorders are unusual following ACA territory infarcts. A minority of patients with small anterior frontal lesions may exhibit asterixis. Hemiparkinsonism has been found with lesions involving the supplementary motor area or cingulate gyrus. Micrographia has also been described with ACA infarcts [164,183]. Involuntary masturbation using the left hand, due to a callosal type of alien hand syndrome and right-sided hemiballismus following bilateral ACA infarcts, highlights the rich semiology of these infarcts [28].
The Middle Cerebral Artery Syndrome
The MCA is the largest branch of the ICA and a continuation of this artery in the direction of the sylvian fissure. The MCA territory is the most common site of ischemic stroke. The clinical syndromes of MCA territory infarction vary according to the site of occlusion (e.g., stem, superior division, inferior division, lenticulostriate branches) and the available collaterals. Clinical features of MCA territory infarction are extremely diverse (e.g., complete MCA territory, deep territory, superficial anterior [superior] territory, and superficial posterior [inferior] territory) [128,218,261,320].
Contralateral weakness affecting the face, the arm, and, to a lesser extent, the leg is a common manifestation of MCA territory infarction. Similarly, contralateral hemisensory loss involving the face, the arm, and, to a lesser extent, the leg is also frequent. Perioral and distal upper limb sensory dysfunction (cheiro-oral syndrome) may occur [43]. Although the cheiro-oral syndrome has been attributed to a lesion of the contralateral postcentral gyrus, it may also be seen with lesions of the contralateral corona radiata [240] or thalamus [158], and even with brainstem lesions [9,125,209,241]. Ataxic hemiparesis with cheiro-oral syndrome may occur with a contralateral posterior capsular infarction [78].
With MCA territory infarction, there may be paresis and apraxia of conjugate gaze to the opposite side, with transient tonic deviation of the eyes and head toward the side of the lesion. Infarcts in the dominant hemisphere for language can be followed by Broca’s, Wernicke’s, conduction, or global aphasia, depending on the site and extent of involvement. Alexia with agraphia may occur with the involvement of the left angular gyrus. Combinations of finger agnosia, acalculia, right–left disorientation, and agraphia (Gerstmann’s syndrome) may be encountered. Infarction in the nondominant hemisphere causes inattention, neglect, denial, apractic syndromes, and impaired prosody. Rarely, nondominant infarction may cause an acute confusional state and acute agitated delirium with affective and autonomic excitement, delusions, and hallucinations [226]. Lesions of either hemisphere may give rise to contralateral homonymous hemianopia or contralateral homonymous inferior quadrantanopia. Cataleptic posturing in isolation from other manifestations of the catatonic syndrome has been mentioned in association with MCA territory infarction [271].
Occlusion of the superior division of the MCA causes contralateral hemiparesis with predominant involvement of the upper extremity and face, hemisensory loss, and conjugate gaze deviation. Visual fields tend to be spared. Superior division of the dominant MCA infarcts causes nonfluent aphasia. A confusional state, aprosodia, contralateral hemi-inattention, and anosognosia are prevailing features of superior division of the right MCA territory infarctions. Frontal cortical infarcts have rarely been associated with transcortical sensory aphasia [161,280], or ataxic hemiparesis [107]. Parietal lobe infarcts may cause the posterior variant alien hand syndrome [160,181].
Wernicke’s aphasia occurs with inferior division of the dominant MCA infarcts. Inferior division of the MCA infarcts of either hemisphere produces homonymous hemianopia or quadrantanopia. Right inferior division of the MCA infarcts may also cause left visual neglect. Temporal lobe involvement can cause an agitated and confused state.
Strokes restricted to the insular cortex have been associated with somatosensory deficits; gustatory disorders; vestibular-like manifestations; cardiovascular disorders including arterial hypertension and arrhythmias; and language and neuropsychological disorders including aphasia, dysarthria, and somatoparaphrenia [65,102,314]. While laterality of autonomic function in the insular cortex remains controversial, insular strokes have also been associated with an increased risk of myocardial injury, cardiac arrhythmias, and sudden death [19,70].
Occlusion of the lateral striate branches of the MCA causes striatocapsular infarction with the involvement of the rostral aspect of the head of the caudate, the anterior limb of the internal capsule, and the putamen (a comma-shaped area on CT scan or MRI) [93]. Clinical manifestations include hemiparesis, affecting mainly the upper limb, and “cortical” abnormalities (aphasia, neglect, and dyspraxia). Less frequently, a pure motor hemiparesis with minimal cortical signs may be seen and, rarely, subtle changes such as dysarthria alone or upper limb clumsiness may occur. Causes of striatocapsular infarction include cardioembolic disease and occlusive vascular disease, more often in the internal carotid than in the MCA [93].
The centrum ovale, which contains the core of the hemispheric white matter, receives its blood supply from the superficial (pial) MCA system through perforating medullary branches, which course toward the lateral ventricles. Patients with infarcts involving the centrum ovale limited to the territory of the perforating medullary branches without the involvement of the lenticulostriate territory often have large infarcts associated with severe disease of the ipsilateral carotid artery and with acute neurologic–neuropsychological impairment no different from that with large MCA infarction. Small infarcts are associated with hypertension or diabetes and with “lacunar syndromes,” usually of progressive onset [49].
Double (multiple) infarcts of the MCA territory of the dominant hemisphere may result in global aphasia without hemiparesis [307], hemianopic hemiplegia without sensory impairment [42], or conduction aphasia with hemiparesis [42]. Bilateral supranuclear facio-pharyngo-glossomasticatory paresis with automatic-voluntary dissociation (Foix-Chavany-Marie syndrome) may also result from bilateral anterior opercular infarcts [269]. Moreover, bilateral temporal infarcts may result in cortical deafness or a Klüver-Bucy syndrome [73].
Malignant MCA territory infarcts resulting from space occupying lesions, often due to an occlusion of the proximal MCA (M1 segment), are associated with an 80% mortality rate [123,128,140]. Occlusion at the origin of the MCA may result in severe flaccid hemiparesis/hemiplegia, contralateral homonymous hemianopia, hemianesthesia, conjugate gaze deviation, pupillary dilatation, and progressive decrease in the level of alertness [140,271]. Neurological deterioration may occur independent from raised intracranial pressure [255]. Global aphasia occurs if the left MCA is occluded. Occlusion of the right MCA produces left body neglect, and bilateral eyelid ptosis. Eyelid ptosis may be an early sign of herniation in large hemispheric infarcts and attributed to upper brainstem involvement [40,171].
VERTEBROBASILAR ARTERY SYNDROMES OF THE BRAINSTEM AND CEREBELLUM
The dominant arterial territories of the brainstem and cerebellum have been carefully delineated by Tatu et al. [298]. The main arterial trunks supplying the brainstem include the vertebral artery, anterior spinal artery, PICA, basilar artery, AICA, SCA, PCA, posterior communicating artery, and AChA The cerebellar arterial supply on its lower half originates from the PICAs and the AICAs, while the superior half of the cerebellum is irrigated by the SCA.
Connected to the brainstem by three pairs of cerebellar peduncles, the main symptoms of cerebellar infarction include vertigo, dizziness, nausea, vomiting, gait unsteadiness, limb clumsiness, headache, dysarthria, diplopia, and decreased alertness. Most prominent signs include limb and gait ataxia, dysarthria, nystagmus, and altered mental status [36].
The areas of the cerebellum supplied by the PICA are variable. The PICA vascularizes the inferior vermis and the inferior and posterior aspects of the cerebellar hemispheres. There are several different patterns of PICA territory cerebellar infarctions. If the medial branch territory is affected, involving the vermis and vestibulocerebellum, the clinical findings include prominent vertigo, ataxia, and nystagmus. If the lateral cerebellar hemisphere is involved, patients can have vertigo, gait ataxia, limb dysmetria and ataxia, nausea, vomiting, conjugate or dysconjugate gaze palsies, miosis and dysarthria. If the infarction is large, lethargy may occur. Hydrocephalus or herniations may develop. With a cerebellar pressure cone (tonsillar hernia), there is downward displacement of the cerebellar tonsils through the foramen magnum, resulting in hemorrhagic necrosis of the cerebellar tonsils and grooving of the ventral surface of the medulla oblongata. Clinical manifestations may include neck stiffness, cardiac and respiratory rhythm disturbances, and apnea. With ascending transtentorial herniation (upward herniation syndrome), there is upward displacement of the superior aspect of the cerebellar hemisphere through the free edge of the tentorial incisura, resulting in midbrain compression. Clinical manifestations include lethargy, coma, paralysis of upward gaze, midposition and unreactive pupils, and abnormal extensor posturing. There is also a syndrome of combined dorsolateral medullary and cerebellar infarction that may be caused by a vertebral artery occlusion or a medial PICA occlusion. Although a PICA occlusion can be the cause of Wallenberg (lateral medullary) syndrome, this syndrome is more often caused by an intracranial vertebral artery occlusion.
The AICA syndrome causes a ventral cerebellar infarction. This artery vascularizes the anterior surface of the simple, superior and inferior semilunar lobules and flocculus, as well as the middle cerebellar peduncle and often the lower aspect of the pontine tegmentum. The signs and symptoms include vertigo, nausea, vomiting, and nystagmus caused by involvement of the vestibular nuclei. There may be ipsilateral facial hypalgesia and thermoanesthesia and corneal hypesthesia because of involvement of the trigeminal spinal nucleus and tract. Ipsilateral deafness and facial paralysis occurs because of involvement of the lateral pontomedullary tegmentum. An ipsilateral Horner syndrome is due to compromise of the descending oculosympathetic fibers. Contralateral trunk and extremity hypalgesia, and thermoanesthesia is caused by involvement of the lateral spinothalamic tract. Finally, ipsilateral ataxia and asynergia is caused by involvement of the cerebellar peduncle and cerebellum. Infarcts in the distribution of the AICA may be forerunners of a basilar artery occlusion [6].
The SCA vascularizes the superior half of the cerebellar hemisphere and vermis, dentate nucleus, and upper aspect of the pontine tegmentum. Infarction in the territory of the SCA produces a dorsal cerebellar syndrome. Vertigo is less common with SCA infarcts than with other cerebellar stroke syndromes. Nystagmus is caused by the involvement of the medial longitudinal fasciculus and the cerebellar pathways. An ipsilateral Horner syndrome is produced by involvement of the descending oculosympathetic tract. Ipsilateral ataxia and asynergia and gait ataxia are caused by the involvement of the superior cerebellar peduncle, brachium pontis, superior cerebellar hemisphere, and dentate nucleus. There is an intention tremor caused by the involvement of the dentate nucleus and superior cerebellar peduncle. Choreiform dyskinesias may be present ipsilaterally. Contralaterally, there is hearing loss caused by lateral lemniscus disruption and trunk and extremity hypalgesia, and thermoanesthesia caused by spinothalamic tract involvement. Patients with SCA territory infarction may also experience ocular contrapulsion (eyes pushed away from side of the lesion) [257].
The midbrain is vascularized by paramedian basilar artery branches, mesencephalic PCA branches, superior cerebellar artery branches, and posterior choroidal artery branches [44,281]. The midbrain contains the nuclei for the oculomotor (III), trochlear (IV), and portions of trigeminal (V) complex. Weber’s syndrome is caused by infarction in the distribution of the penetrating branches of the PCA affecting the cerebral peduncle, especially medially, with damage to the fascicle of CN III and the pyramidal fibers. The resultant clinical findings are contralateral hemiparesis caused by corticospinal and corticobulbar tract involvement and ipsilateral oculomotor paresis, including a dilated pupil. A slight variation of this syndrome is the midbrain syndrome of Foville in which the supranuclear fibers for horizontal gaze are interrupted in the medial cerebral peduncle, causing a conjugate palsy to the opposite side. Benedikt’s syndrome is caused by a lesion affecting the mesencephalic tegmentum in its ventral portion, with the involvement of the red nucleus, brachium conjunctivum, and fascicle of CN III. This syndrome is caused by infarction in the distribution of the penetrating branches of the PCA to the midbrain. The clinical manifestations are an ipsilateral third nerve paresis, usually with pupillary dilation, and a contralateral hemitremor, hemiathetosis, or hemichorea. Claude’s syndrome (featuring elements of both Benedikt’s and Nothnagel’s syndromes) is caused by lesions that are more dorsally placed in the midbrain tegmentum than in Benedikt’s syndrome. There is injury to the dorsal red nucleus, which results in more prominent cerebellar signs (asynergia, ataxia, dysmetria, and dysdiadochokinesia) without the involuntary movements. Nothnagel’s syndrome is characterized by an ipsilateral third nerve paresis with contralateral cerebellar ataxia. Nothnagel’s syndrome is caused by a lesion in the area of the superior cerebellar peduncle, in the distribution of the penetrating branches of the PCA to the midbrain, and may represent a variant of the dorsal midbrain syndrome [201]. Parinaud’s (dorsal midbrain syndrome, pretectal syndrome, Sylvian aqueduct syndrome) syndrome can result from infarctions in the midbrain territory of the PCA penetrating branches. This syndrome is characterized by supranuclear paralysis of vertical gaze, defective convergence, spasm/ paresis of accommodation, convergence–retraction nystagmus, light-near dissociation of the pupils, lid retraction (Collier’s sign), and skew deviation.
Pure motor hemiparesis, four-limb ataxia, and hypesthesic ataxic hemiparesis caused by midbrain lesions are discussed with lacunar syndromes. Other infarctions in the distribution of the penetrating branches of the PCA to the midbrain may be characterized by nuclear oculomotor palsy, unilateral or bilateral internuclear ophthalmoplegia, pseudoabducens palsy, and locked-in syndrome [61]. Parkinsonism and micrographia have rarely been observed in patients with midbrain and thalamomesencephalic strokes [177].
Atherothrombotic disease in the vertebrobasilar system has a predilection for the distal vertebral artery and the lower or middle basilar artery [274]. Atherosclerotic involvement of the intracranial portion of the vertebrobasilar system frequently occurs in tandem with and is the common pathologic mechanism associated with the syndrome of vertebrobasilar infarction. Top of the basilar or rostral basilar artery syndrome [61] is caused by infarction of the midbrain, thalamus, hypothalamus, paramedian diencephalon, medial temporal lobes and occipital lobes [215]. It is caused by occlusive vascular disease, often embolic in nature of the rostral basilar artery. The following signs may occur:
Behavioral abnormalities include coma, akinetic mutism, hypersomnolence, memory disturbances, or agitated delirium. Peduncular hallucinosis, reported with focal lesions of the cerebral peduncles or with bilateral involvement of the medial aspect of the substantia nigra pars reticulata and characterized by complex, nonthreatening visual hallucinations, may also be present [114,212].
Ophthalmologic findings include unilateral or bilateral paralysis of upward or downward gaze, impaired convergence, pseudoabducens palsy, convergence–retraction nystagmus, abnormalities of ocular abduction, Collier’s sign, skew deviation, and oscillatory eye movements. Visual disorders that may be present include homonymous hemianopia or quadrantanopia, cortical blindness, and Balint’s syndrome characterized by psychic paralysis of gaze, simultanagnosia, and optic ataxia [225]. Alexia without agraphia may be seen with dominant occipital lesions. Bilateral lesions may produce visual agnosia or prosopagnosia. Pupillary abnormalities include small and reactive pupils, large or midposition and fixed pupils, and occasionally midbrain corectopia characterized by eccentric or oval pupils.
Motor and sensory deficits may likewise occur. Although there are many named classic pontine syndromes (e.g., Millard-Gubler, Raymond, Foville’s, Raymond-Cestan, Marie-Foix, and Brissaud), the most useful categorization is based on neuroanatomical divisions (ventral, tegmental, and bilateral) [27]. Pontine infarcts can cause pure motor hemiparesis, sensorimotor stroke, ataxic hemiparesis, dysarthria– clumsy hand syndrome, ataxic tetraparesis, or bilateral cerebellar ataxia [194]. Pontine infarctions may produce combined motor, sensory, cerebellar, and cranial nerve dysfunction. The pons contains the nuclei for the abducens (CN VI), facial (CN VII), vestibulocochlear (CN VIII), and a portion of the nuclei of the trigeminal (CN V) nerve. Locked-in syndrome (“ventral pontine syndrome” or “de-efferented state”) is the result of bilateral destruction usually at the level of the basis pontis involving the rostral and middle pontine segments interrupting the descending corticobulbar and corticospinal tracts, causing quadriplegia, aphonia, anarthria, and impairment of the horizontal eye movements. Wakefulness is maintained because of sparing of the ascending reticular formation. The patient can move his or her eyes vertically and can blink because the supranuclear ocular motor pathway lies more dorsally. Pupillary reactivity is spared. Respiratory function remains intact. Most cases are due to thrombotic or embolic occlusion of the perforating paramedian branches of the basilar artery. In some patients, there is a “heralding” hemiparesis that may be misleading, making the lesion seem cortical in nature. However, within a few hours, there is progression to bilateral hemiplegia and cranial nerve findings associated with the locked-in syndrome [247]. Pathologic laughter (Fou rire prodromique) may herald the development of a brainstem stroke as a result of basilar artery occlusion [119]. Pure motor hemiparesis and ataxic hemiparesis caused by pontine lesions are discussed with lacunar syndromes.
Occlusion of the AICA can lead to the lateral inferior pontine syndrome. Findings associated with this syndrome include ipsilateral facial paralysis, impaired facial sensation, paralysis of conjugate gaze to the side of the lesion, deafness, tinnitus, and ataxia. Contralateral to the lesion, there is hemibody impairment to pain and temperature, which in some instances includes the face. There may be horizontal and vertical nystagmus and oscillopsia. Bilateral sudden deafness may be the heralding manifestation of an anterior inferior cerebellar artery infarction [195]. The medial inferior pontine syndrome is caused by occlusion of a paramedian branch of the basilar artery. With this syndrome, there is ipsilateral paralysis of conjugate gaze to the side of the lesion, abducens nerve palsy, nystagmus, and ataxia. Contralateral to the lesion, there is hemibody impairment of tactile and proprioceptive sensation and paralysis of the face, arm, and leg. An occlusion of the AICA may lead to the total unilateral inferior pontine syndrome, a combination of the symptoms and signs seen with the lateral and medial pontine syndromes.
The lateral pontomedullary syndrome can occur with the occlusion of the vertebral artery.
The manifestations are a combination of the medial and lateral inferior pontine syndromes. Occlusion of the paramedian branches of the midbasilar artery can lead to ipsilateral impaired sensory and motor function of the trigeminal nerve with limb ataxia, characteristics of the lateral midpontine syndrome. Ischemia of the median pontine region is caused by occlusion of the paramedian branch of the midbasilar artery and can lead to ipsilateral limb ataxia. Contralateral to the lesion, eye deviation and paralysis of the face, arm, and leg occur. Although there are predominant motor symptoms, which predominate in the upper extremity because of the somatotopic organization of the corticospinal tract in the basis pontis, variable impaired touch and proprioception may also occur. Paramedian pontine base lesions may also result in dysarthria. The lateral superior pontine syndrome may occur with the occlusion of the superior cerebellar artery and produces a characteristic ipsilateral Horner syndrome, horizontal nystagmus, paresis of conjugate gaze, occasional deafness, and severe gait and limb ataxia. Contralateral to the lesion, there is hemibody impaired sensation to pain and temperature, skew deviation, and impaired tactile, vibratory, and proprioceptive sensation in the leg greater than in the arm.
Pontine infarctions may also produce transient pathologic crying and laughter [177], horizontal gaze abnormalities including abducens nerve palsy, INO, horizontal gaze palsy, a one-and-a-half syndrome [155], transient upbeat nystagmus [196], hemi-seesaw nystagmus [72], loss of vertical saccades and pursuit with horizontal gaze palsy [193], numbness and hypesthesia of the midline facial region [207], trigeminal sensory neuropathy and neuralgia [142,156,182], isolated volitional type of facial palsy [305,311], ipsilateral facial spasm and contralateral hemiparesis [325], hemimasticatory spasm [122], body lateropulsion [333], anosognosia for hemiplegia [18], and unilateral hyperhidrosis [248]. The medulla oblongata contains the nuclei for the glossopharyngeal (CN IX), vagus (CN X), and hypoglossal (CN XII), as well as portions of the trigeminal (CN V) nuclei, vestibulocochlear (CN VIII), and spinal accessory (CN XI) nerves. The lateral medullary syndrome (Wallenberg syndrome) is most often caused by atherosclerotic occlusion or dissection of the intracranial segment of the vertebral artery. Less commonly, it is caused by occlusion of PICA, small vessel infarction or cardiac embolism [166]. Dissections were more frequent with caudally placed medullary lesions. Depending on the extent of the medullary damage, clinical findings vary considerably [80]. Wallenberg syndrome consists of a constellation of signs and symptoms including ipsilateral limb and gait ataxia with a tendency to fall to the ipsilateral side (body lateropulsion) due to involvement of the restiform body and inferior surface of the cerebellar hemisphere. There is ipsilateral facial hypalgesia and thermoanesthesia because of involvement of the descending tract and nucleus of the trigeminal nerve. There is paresis of the pharyngeal muscles with palatal weakness, decreased gag reflex, dysphagia, and dysphonia due to ipsilateral vocal cord paresis caused by the involvement of the nucleus ambiguus. An ipsilateral Horner syndrome is present because of compromise of the descending oculosympathetic pathways. Contralateral trunk and extremity hypalgesia and thermoanesthesia occurs caused by involvement of the spinothalamic tract. These patients experience vertigo and often an illusionary tilting of the environment by 90 to 180 degrees. Nystagmus and a host of oculomotor symptoms may be caused by compromise of the ipsilateral vestibular nuclei or functional compromise of the fastigial nucleus. Patients may demonstrate a horizontal rotatory jerk nystagmus, beating away from the side of the lesion; the nystagmus either stops or reverses with eye closure [95]. There may be gaze-evoked nystagmus, seesaw nystagmus, impaired contralateral pursuit eye movements, saccadic lateropulsion, ocular lateropulsion [131,180,184,287], skew deviation, and ipsilateral horizontal gaze deviation.
The classic sensory signs of Wallenberg syndrome, due to involvement of the crossed lateral spinothalamic tract and the ipsilateral descending tract and nucleus of the trigeminal nerve, include the loss of pain and heat sensation in the ipsilateral face and contralateral hemibody and extremities. However, numerous variants of this classic sensory pattern have been recognized, including contralateral or bilateral facial sensory changes due to the involvement of the ascending as well as the descending trigeminal fibers, partial involvement of the face, changes in the sensory level of the trunk, sensory changes in the ipsilateral extremities, and hemisensory loss of the contralateral whole hemibody [82,154,173,179,210,317].
As a rare occurrence, some patients with Wallenberg syndrome display ipsilateral facial palsy presumably due to the involvement of an aberrant corticobulbar tract, or extension of the infarct to the pons with compromise of the facial nerve nucleus or fascicles; emotional-facial paresis related to involvement of looping medullary corticofacial projections in the upper medulla [66]; ipsilateral hemiplegia (Opalski syndrome) due to submedullary extension; ipsilateral wild arm ataxia probably related to involvement of the lateral cuneate nucleus [81]; clumsiness of the ipsilateral upper limb resulting from extension of the lesion into the subolivary area [56]; central pain combining thermal hypesthesia with thermal and touch allodynia [253]; isolated contralateral thermoanesthesia of the trunk and limbs from involvement of the dorsal portion of the lateral spinothalamic tract [10]; and loss of taste resulting from involvement of the nucleus and tractus solitarius. Contralateral hyperhidrosis and ipsilateral anhidrosis can also be observed in the late phase of patients with the Wallenberg syndrome and is likely due to a lesion of the sympathetic pathway [265]. Hiccough has been attributed to involvement of the respiratory centers in the medullary reticular formation.
The medial medullary syndrome (Dejerine’s syndrome) is less common and may be caused by distal atherosclerotic vertebral artery occlusive disease [173,318]. Vertebral artery dissection, dolichoectasia of the vertebrobasilar system, or embolism are less common causes of the medial medullary infarction. The findings associated with this syndrome include an ipsilateral lower motor neuron paralysis of the tongue and contralateral paralysis of the arm and leg. The face is often spared. An ipsilateral lingual palsy is seen in only half of the cases. In rare instances, upbeat nystagmus may be present [173]. A crossed motor hemiparesis (hemiplegia cruciata) is an extremely rare occurrence [39]. In addition, there is contralateral loss of tactile, vibratory, and position sense. These signs are attributed to the involvement of the pyramidal tract rostral to their decussation, the fibers and nucleus of the hypoglossal nerve, and the medial lemniscus [26,306]. As a rare occurrence, some patients with medial medullary infarcts display triparesis probably due to a presumptive rostral decussation of lower extremity fibers [110] or isolated acute bilateral tongue paralysis due to exclusive and simultaneous involvement of the hypoglossal nerves at the medullary tegmentum [30]. The occlusion of the vertebral artery can lead to a total unilateral hemimedullary (Babinski-Nageotte) syndrome, which is a combination of the medial and lateral medullary syndrome. Bilateral medial medullary and bilateral lateral medullary syndromes are extremely rare. Because of the separate arterial topography supplying the medulla, the simultaneous occurrence of ischemic lesions involving the lateral and medial parts of the medulla is extremely rare [112,229].
THE POSTERIOR CEREBRAL ARTERY SYNDROME
In the majority of people, the two PCAs are the terminal branches of the basilar artery, but in 20%–25%, one of the PCAs may originate from the ICA via a posterior communicating artery. The clinical picture of PCA territory infarction varies according to the site of occlusion and the availability of collaterals. Occlusion of the precommunal P1 segment causes midbrain, thalamic, and hemispheric infarction. Occlusion of the PCA in the proximal ambient segment before branching in the thalamogeniculate pedicle causes lateral thalamic and hemispheral symptoms. Occlusions may also affect a single PCA branch, primarily the calcarine artery, or cause a large hemispheric infarction of the PCA territory. Whether embolic, thrombotic, migrainous, or due to intrinsic atherosclerotic disease, partial syndromes of the PCA are the rule [192]. Another cause of PCA infarcts is compression of the artery against the tentorium during uncal herniation [263]. Infarction in the distribution of the hemispheric branches of the PCA may produce contralateral homonymous hemianopia caused by infarction of the striate cortex, the optic radiations, or the lateral geniculate body. There is partial or complete macular sparing if the infarction does not reach the occipital pole. The visual field defect may be limited to a quadrantanopia. A superior quadrantanopia is caused by infarction of the striate cortex inferior to the calcarine fissure or the inferior optic radiations in the temporo-occipital lobes. An inferior quadrantanopia is the result of an infarction of the striate cortex superior to the calcarine fissure or the superior optic radiations in the parieto-occipital lobes.
More complex visual changes may occur, including formed or unformed visual hallucinations, visual and color agnosias, or prosopagnosia. Finally, some alteration of sensation with PCA hemispheral infarctions occurs, including paresthesiae, or altered position, pain, and temperature sensations. Sensory findings may indicate thalamic ischemia due to occlusion of the precommunal or proximal postcommunal segments of the PCA, thalamoparietal ischemia due to occlusion of the more distal PCA or its parieto-occipital branches, or brainstem ischemia caused by vascular occlusive disease in the proximal vertebrobasilar arterial system [115]. Infarction in the distribution of the callosal branches of the PCA involving the left occipital region and the splenium of the corpus callosum produces alexia without agraphia (pure word blindness), occasionally associated with color anomia and object and photographic anomia [87]. In this syndrome, patients can write, speak, and spell normally but are unable to read words and sentences. The ability to name letters and numbers may be intact, but there can be inability to name colors, objects, and photographs. Right hemispheric PCA infarctions may cause contralateral visual field neglect. Amnesia may present with PCA infarctions that involve the left medial temporal lobe or when there are bilateral mesiotemporal infarctions [61,106,214,250,251]. In addition, an agitated delirium may occur with unilateral or bilateral penetrating mesiotemporal infarctions [89]. Large infarctions of the left posterior temporal artery territory may produce an anomic or transcortical sensory aphasia. Infarctions in the distribution of the penetrating branches of the PCA to the thalamus can cause aphasia if the left pulvinar is involved, akinetic mutism, global amnesia, and the Dejerine-Roussy syndrome.
Occlusion of the calcarine artery may be associated with pain in the ipsilateral eye [266]. Bilateral infarctions in the distribution of the PCA may cause bilateral homonymous hemianopia. Bilateral occipital or occipitoparietal infarctions may result in cortical blindness with preserved pupillary reflexes. Patients often deny or are unaware of their blindness (Anton’s syndrome). Bilateral altitudinal visual field defects seldom result from bilateral occipital lobe infarcts [202]. Infarction in the territory of the hemispheric branches of the PCA may also be accompanied by formed or unformed visual hallucinations (“release hallucinations”) [59], visual and color agnosias, or prosopagnosia (agnosia for familiar faces). Apraxia of ocular movements is often present with bilateral lesions. Some patients with bilateral occipital or parieto-occipital infarctions have a Balint syndrome. Proximal PCA occlusion may simulate MCA occlusion when it causes hemiparesis, hemianopsia, hemispatial neglect, aphasia, and sensory loss or inattention [68]. “Cortical” signs are probably explained by thalamic involvement.
SYNDROMES OF THALAMIC INFARCTION
The thalamus is the largest subdivision of the diencephalon. The main thalamic blood supply originates from the posterior communicating arteries and the perimesencephalic segment of the PCA. Thalamic infarctions typically involve one of the four major vascular regions (Fig. 22.4): posterolateral, anterior, paramedian, and dorsal [51,275,281]. Posterolateral thalamic infarctions result from occlusion of the thalamogeniculate branches arising from the P2 segment of the PCA. Three common clinical syndromes may occur: pure sensory stroke, sensorimotor stroke, and the thalamic syndrome of Dejerine-Roussy. In the latter syndrome, the patient has contralateral sensory loss to all modalities, with occasional sparing of the face because of the more medial location of the nucleus ventralis posteromedialis, severe dysesthesias of the involved side (thalamic pain), vasomotor disturbances, transient contralateral hemiparesis, and mild choreoathetoid or ballistic movements. The pain or involuntary movements may present weeks or months after the stroke. Anterior thalamic infarction results form occlusion of the polar or tuberothalamic artery. The main clinical manifestations consist of fluctuating levels of consciousness, abulia, apathy, disorientation, lack of insight and personality changes, contralateral emotional-facial paresis, occasional hemiparesis, and visual field deficits. Left-sided infarcts are associated with language deficits (thalamic aphasia, dysprosody, dysarthria); selective impairment in semantic memory may be seen [279], whereas hemineglect, alien hand syndrome, and visual spatial deficits [205,242] may be seen primarily in patients with right-sided lesions. Paramedian thalamic infarctions result from occlusion of the paramedian or thalamic and subthalamic arteries (thalamoperforating pedicle). Main clinical manifestations include somnolence or transient loss of consciousness, memory loss or mood disturbances, and vertical gaze abnormalities. Paramedian thalamic infarcts may also produce abnormal sleep and body core temperature abnormalities [224] and bilateral eyelid tremor on voluntary eyelid closure [152]. Paramedian thalamic infarcts may be unilateral or bilateral, and often result from an embolic occlusion of the basilar apex, causing a disconnection between the thalamus and the frontal lobes. Bilateral paramedian thalamic infarcts are rare; a venous etiology is seldom responsible. These infarcts may result in hypersomnolence, marked memory impairment with perseveration and confabulation, akinetic mutism, acute dementia [185], lexical semantic deficits [90], and hypersexuality [231]. A rare variant, named the artery of Percheron, is a solitary trunk arising from one of the proximal segments of the PCA, and supplies the paramedian thalami and rostral midbrain bilaterally. Occlusion of this artery results in bilateral medial thalamic infarcts [185,208,249].
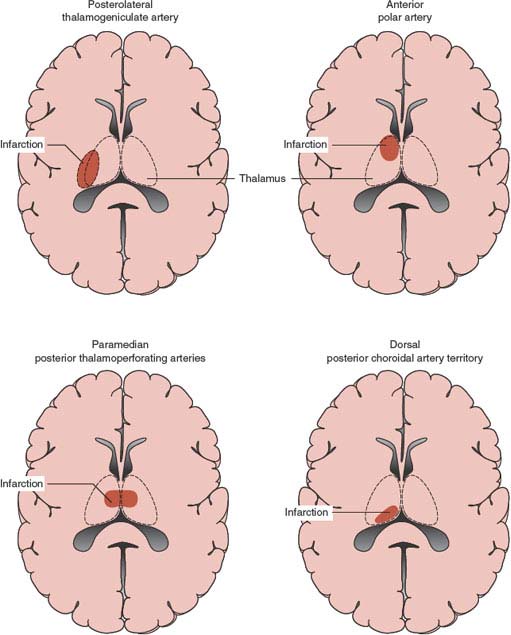
FIG. 22.4. Patterns of thalamic infarction.









