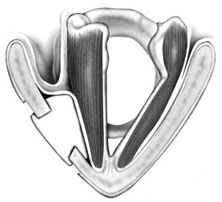
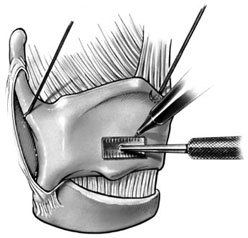
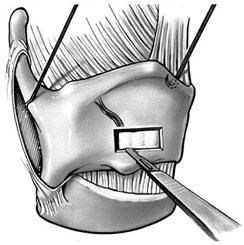
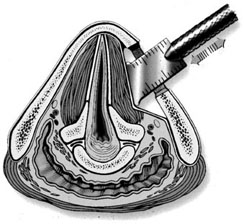
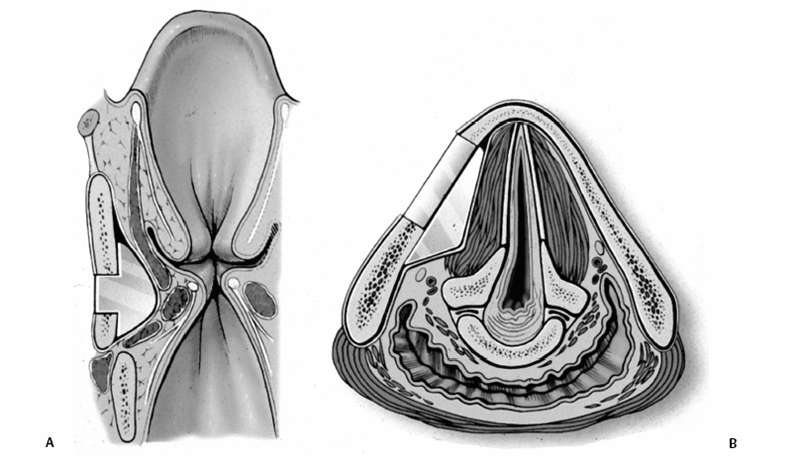
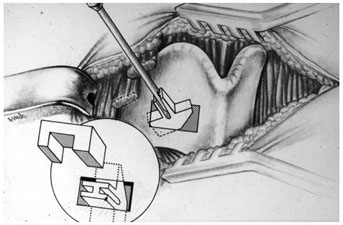
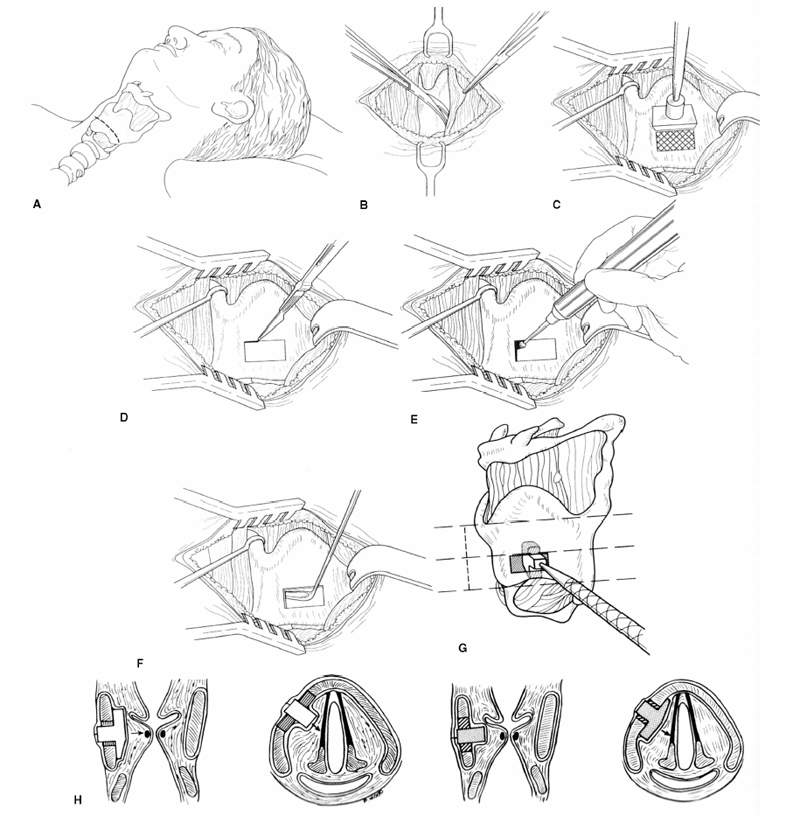
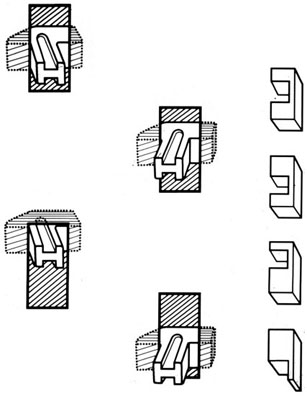
Chapter 15 Restoration of vocal function with laryngeal framework surgery (laryngoplastic phonosurgery) was introduced at the beginning of the 20th century. Today, these procedures have emerged as the dominant surgical management approach for the treatment of the aerodynamic incompetence and acoustic deterioration associated with vocal fold paralysis/paresis. Other indications include cancer defects, vocal fold scar, sulcus vocalis, bowing associated with vocal fold atrophy, laryngeal trauma, and neuromuscular disorders including abductor spasmodic dysphonia and parkinsonism. Laryngeal framework surgery has also been employed to alter pitch for gender reassignment; however, this topic is not discussed here. Although medialization of the musculomembranous vocal fold by means of rearranging the laryngeal cartilage framework was described by Payr1 in 1915, and others in the mid–20th century,2,3 Isshiki et al4–6 championed the systematic analysis and laryngoplastic treatment of glottal incompetence in the 1970s. He designed his medialization procedure of the musculomembranous vocal fold with the use of a synthetic implant in 1974. In 1978, Isshiki et al5 designed the arytenoid adduction procedure to treat patients with large glottal gaps secondary to a malpositioned arytenoid. One of his outstanding contributions is that he taught surgeons that laryngeal framework procedures could be done with facility utilizing local anesthesia with sedation. The concept that the cricoarytenoid joint could be dissected and manipulated under local anesthesia to allow for phonatory feedback was revolutionary. Based on this seminal work, the adduction arytenopexy2,7–9 and cricothyroid subluxation7,8,10 procedures were introduced to further enhance phonatory reconstruction. Isshiki6 described four basic surgical procedures that he termed thyroplasty types I to IV for altering the conformation of the thyroid cartilage and the arytenoid adduction, which attempts to close the posterior (cartilaginous) glottis. Thyroplasty type I (Fig. 15.1) is the most widely used of Isshiki’s original thyroplasty techniques. It involves creating a rectangular cartilaginous window at the level of the true vocal fold and using cartilage, Silastic, Gore-Tex, or other implant material to medialize the true vocal fold. This procedure achieves closure of the musculomembranous vocal fold only; arytenoid position is not appreciably altered by the implant. Thyroplasty type I is a relatively simple and reversible procedure that is ideally performed with local anesthesia to facilitate fine-tuning of the voice with precise placement of the implant material. There have been a large number of manuscripts describing a multitude of variations of the original procedure, primarily introducing different implant materials and their placement. As thyroplasty type I does not primarily influence arytenoid position, this procedure is often coupled with an arytenoid adduction or arytenopexy to close both the anterior (musculomembranous) and posterior (cartilaginous) glottis. Fig. 15.1 A small implant is placed lateral to the inner perichondrium of the thyroid lamina. Thyroplasty type II is a procedure in which the posterolateral thyroid lamina is lateralized; there are few indications for its use. Thyroplasty type III is used to lower the vocal pitch by shortening the anteroposterior (AP) dimension of the glottis. The primary function is to release vocal fold tension to lower pitch. Conversely, thyroplasty type IV increases the AP dimension of the glottis, thereby increasing the tension on the vocal folds and raising the vocal pitch. Thyroplasty types III and IV have been used in gender reassignment surgery to bring the fundamental frequency into the normal range for the newly assigned sex. Arytenoid adduction was described by Isshiki et al5 as a way of mimicking the medializing effect of the lateral cricoarytenoid muscle on the vocal process. A paralyzed arytenoid tends to fall forward and laterally on the cricoid facet, shortening the AP length of the vocal fold and moving the arytenoid away from the midline. The classic arytenoid adduction procedure is performed under local anesthesia with sedation by exposing the posterior aspect of the thyroid lamina. The cricoarytenoid joint is identified, and a suture is placed through the muscular process of the arytenoid and passed anteriorly through the thyroid lamina, thereby rotating the vocal process medially to meet the opposite vocal process during phonation. Prior to the conclusion of the surgical procedure the position is visually verified by means of a flexible fiberoptic laryngoscope. The ideal procedure(s) to treat aerodynamic glottal incompetence that is associated with paralytic/paretic dysphonia should attempt to simulate the normal vocal fold position during phonation with regard to the following interdependent parameters: (1) position of the musculomembranous region in the axial plane, (2) position of the arytenoid in the axial plane, (3) height of the vocal fold, (4) length of the vocal fold, (5) contour of the vocal fold edge in the musculomembranous region, (6) contour of the vocal fold edge in the arytenoid region, and (7) mass and viscoelasticity of the vocal fold. Furthermore, the procedure(s) ideally should be easy to perform, associated with few complications, reliable, reversible, and not threatening to the airway.11 The basic technique for medialization laryngoplasty is very similar for each of the implant materials. If one understands the fundamental principles behind placing the vocal cord into the physiologic phonating position, the choice of implant material is of secondary importance. One can achieve very good results with almost any implant, if the principles outlined below are adhered to. Some implants due to their size constraints may make it harder to place the vocal fold in the correct position. Other implants offer initial excellent voice results, but are not stable and may move from the initial location with delayed decrease in voice quality. Following the outlined steps in any described technique is secondary to a basic understanding of the physiologic phonating position of the vocal cord. Unless contraindicated, some authors start patients on a Medrol DosePack the day before surgery, and patients are given 0.2 mg/kg of Decadron 1 hour prior to the procedure. This helps to minimize intraoperative swelling, which can interfere with determining the implant size, and reduces postoperative airway swelling. The anesthetic preparation of the patient is extremely important. It is very easy to oversedate the patient, leading to slurred lethargic speech. With slight oversedation the muscle tone of the hypopharynx grows weak, making it very difficult to see the vocal folds on the monitor, or judge voice improvement in the lethargic patient. The majority of patients need only a few milligrams of Versed to undergo the entire procedure. It is critical to have an alert and oriented patient to obtain good results. Some anesthesiologists prefer to administer intravenous propofol, which can be quickly reversed when patient cooperation is necessary, and then the patient can be quickly sedated after implant placement. Once the patient is mildly sedated, the patient’s nose is anesthetized by instilling 4 cc of 5% cocaine into the nasal cavity. A flexible fiberoptic scope is introduced transnasally, suspended above the patient, and attached to the video monitor system so that the larynx can be visualized on the monitor during the entire procedure (Fig. 15.2). Other surgeons pass an endoscope at critical times during the procedure when visualization is important. This makes the patient more comfortable during the procedure. Fig. 15.2 The flexible nasolaryngoscope, suspended above the patient, and attached to the video monitor, is used to view the position of the vocal cord throughout the procedure. Preoperative intramuscular Robinal is administered to decrease the pharyngeal secretions during the procedure. To increase the likelihood of success, one needs both auditory and visual feedback, to access the position of the vocal fold during testing. Intraoperative visualization of the vocal fold provides two distinct advantages: (1) The relative position of the vocal fold within the window can be identified early in the procedure. (2) Initial hyperfunctional, pressed, strained voice, can be very confusing to the surgeon unless he can observe the position of the vocal fold on the monitor. In this setting, with the vocal fold displaced toward the midline, the hyperfunctional state of the larynx can be readily seen on the monitor. As outlined below, the patient is coached to relax his larynx during continued voice testing, until the hyperfunctional state is released. This slow, relaxation of the supraglottic larynx is seen on the monitor as the voice begins to improve. In the rare patient, where the hyperfunctional voice does not break, one can complete the operation, by observing the television monitor and placing the paralyzed vocal fold in the midline. Even though the perioperative voice continues to be strained, the majority of these patients will develop reasonable quality voice. Without the ability to visualize the glottis it would be very difficult to acquire good voice results in this subgroup of patients. Local anesthesia is injected into the skin and subcutaneous tissues of the anterior neck. It is infiltrated along the thyroid cartilage extending deeply to the posterior border of this cartilage. This is usually sufficient to perform the entire procedure. Local anesthesia is never injected into the paraglottic space or the postcricoid region. As long as the mucosa of the endolarynx, and the piriform sinuses, are not violated, the patients will have very little sensation in these two regions. An incision is created in a skin crease overlying the cricothyroid membrane. If an arytenoid adduction is planned, the incision should be at least 5 to 6 cm in length. Initial elevation is performed superficial to the strap muscle layer. If one anticipates atrophy of the thyroarytenoid muscle, then fat is carefully left approximated to the fascia overlying the strap muscles. If needed later in the procedure, this can be developed into a laterally based vascularized fat flap to use in reconstruction of the paraglottic space. After the strap mussels have been separated in the midline, the medial 2 cm of the sternohyoid muscle is divided 6 to 7 mm inferior to its attachment to the hyoid bone. The sternothyroid muscle is lifted up as the perichondrium is elevated off the thyroid cartilage. If the addition of an arytenoid adduction (AA) is anticipated, the perichondrium is elevated to the posterior border of the thyroid cartilage. The window is laid out on the lateral surface of the cartilage. There has been considerable debate over the location of this window. Many formulas have been described to accurately locate this window just lateral to the vocal fold. Most of these formulas result in a window that is too high. The implant, if placed in the center portion of these higher windows, leads to overmedialization of the false vocal fold and the ventricle, resulting in rough or diplophonic voice. The lower the window is, the easier it is to accomplish medialization in the appropriate plane. Therefore, the window is placed as low on the thyroid cartilage as possible, leaving a 2- to 3-mm inferior strip of cartilage below the edge of the window (Fig. 15.3). Fig. 15.3 The window is outlined as low as possible leaving a 2- to 3-mm strut of cartilage below the window. The anterior border of the window is placed back from the anterior commissure 5 mm in women and 7 mm in men. In other descriptions of the surgical technique, the cartilage island is left within the window and displaced into the paraglottic space as a biologic tissue implant. Leaving the island as a natural tissue barrier is not necessary, and in reality the island impedes consistently good voice results. Early in our series we realized that the cartilage island was not stable. The island often shifted out of position, or atrophied, resulting in loss of the initial improvement in voice quality. Also, if the cartilage island is left within the window, and used to displace the vocal fold, the window must be placed in exactly the right location to achieve optimal results. It is also common for the needed plane of medialization to be so discrete that medialization within the superior portion of a 6-mm-wide window results in poor strained voice, whereas medialization within the lower third results in normal voice. It is far more practical to use the window as an entry into the paraglottic space, using an implant system that allows one infinite variability in placing the vocal fold in the physiologic phonating position, no matter where the position of the vocal fold is in relation to the window. Another common mistake is creating a window that is too small. This makes it very difficult to test medialization in all aspects of the paraglottic space. It is also very difficult to place an appropriate-size implant through a small window. In view of this, we recommend creating a window that is it least 6 by 13 mm in size. The window is placed parallel to the imagined plane of the vocal fold as low as possible on the thyroid cartilage, as outlined above. The window is started back from the anterior commissure approximately 5 mm in the female and 7 mm in the male. The window may need to extend to 15 to 16 mm in length in large men to have access to the paraglottic space to appropriately test and medialize the posterior one third of the vocal fold. In a female of small stature, it may be necessary to extend the window toward the anterior commissure of the thyroid cartilage. These intraoperative window adjustments are usually easy to determine as one tests for the location of the vocal fold within the window. A high-speed drill with a 3-mm cutting burr is used to remove the cartilage from the window. The inner perichondrium is carefully protected as the cartilage is removed, to prevent damage of the underlying muscle by the drill bit. The early descriptions of medialization laryngoplasty (ML) recommended preservation of the inner perichondrium as a biologic barrier to prevent implant extrusion. In 1987 we began to remove the entire cartilage island and carefully divide the inner perichondrium to consistently obtain discrete medialization at the level of the vocal fold. We recommend dividing the perichondrium at the edge of the window and removing it from within the window. This can be done carefully with a small sharp knife blade without injuring the underlying thyroarytenoid (TA) muscle fascia. As the perichondrium is divided, one can see the natural space between the perichondrium and the TA muscle fascia (Fig. 15.4). The vascular supply of the paraglottic space runs deep to the TA fascia; therefore, this potential space between the two layers can be quite dry and avascular. At this point one carefully elevates the paraglottic soft tissue away from the inner perichondrium, leaving the inner perichondrium still in place attached to the medial surface of the thyroid cartilage. The position of the vocal fold in relation to the window can now be ascertained by gently displacing the TA muscle with a blunt probe in all quadrants of the window while observing the soft tissue movement on the monitor. Displacement of the soft tissue in the superior half of the window often results in prolapse of the ventricle and or the false vocal fold. In most men, the maximum plane of medialization, necessary to obtain quality voice, is in the lower third of the window. Even in females, with the window placed near the lower border of the cartilage, the plane of medialization is usually in the middle to lower half of the window. Determination of the appropriate plane of maximum medialization (the level within the window) is critical in obtaining good voice. One of the major causes of poor voice results, leading to revision surgery, is an appropriately sized implant that is placed 2 to 3 mm too high. This results in rough raspy voice quality, with marked vocal fatigue. Fig. 15.4 To prepare for implant placement, the inner perichondrium is divided around the window edge. The plane of elevation is medial to the inner perichondrium, and superficial (lateral) to the thyroarytenoid muscle fascia. To judge the size of the implant the voice is tested during medialization of the focal fold with the depth gauge. The size and shape of a depth gauge should simulate the medialization that will be obtained with the implant (Fig. 15.5). Due to the smaller size and more obtuse angle of the thyroid cartilage in females, a smaller depth gauge is needed for the female larynx. The voice is tested by moving the depth gauge in all aspects of the window with varying degrees of medialization. The medialization of the vocal fold is observed on the television monitor as this testing occurs. Fig. 15.5 The depth gauge is used within the window for intraoperative voice testing. The testing gauge should simulate the shape of the implant. The majority of patients who present with vocal cord paralysis have developed compensatory speech patterns with a hyperfunctional pressed voice quality. During the initial aspect of testing, as the paralyzed vocal fold is displaced toward the midline this hyperfunctional, pressed voice quality only becomes worse. Instead of the beautiful voice the surgeons hope to hear, it is very common for the patient to develop an extremely strained voice during the initial aspect of testing. When this occurs, the surgeon, while observing the position of the vocal fold on the monitor, depresses and holds it as near the midline as possible. The patient is then coached to relax and try to speak in a softer voice, releasing the strained quality. It may take anywhere from 2 to 20 minutes of continued coaching for this hyperfunctional speech pattern to break. With a persistent, stubborn, pressed voice, the patient is asked to start each word by humming, which relaxes the larynx. The patient is instructed to count to 10, starting each word with the humming sound. In the majority of patients this hyperfunctional speech breaks within 5 to 10 minutes. It takes patience on the part of the surgeon to wait for a good voice to develop. After the pressed voice begins to break, and reasonable voice quality develops, one can then judge the size of the implant needed by the position of the depth gauge within the window. The average depth of medialization in most men and women is approximately 5 mm in the posterior aspect of the implant, with 1 mm or less medialization needed in the anterior aspect of the window. An implant is fashioned matching the measurements that produced excellent voice during testing with the depth gauge. The lower flange is placed into the window first as the implant is rotated and compressed through the window. The flanges and lateral strut lock the implant into position (Fig. 15.6). A 4-0 Prolene suture placed through the implant around the lower strut of the window further stabilizes the implant. As one gains experience with this procedure, it is impressive how subtly different patients are. To achieve consistently good voice results, one must tailor the size of the implant and the level of medialization to each patient. As can be realized from the above discussion, starting with a limited selection of prefabricated implants can compromise the voice results in some patients. Fig. 15.6 The final implant is placed within the pocket just deep to the thyroid cartilage displacing the thyroarytenoid (TA) muscle toward the midline. The implant is carved with wide flanges to fit snugly against the inner surface of the thyroid cartilage, creating a stable implant that resists movement or migration. Rationale The VoCoM thyroplasty method12 is a self-contained system of implants and instruments to allow accurate vocal fold medialization. The implants come in five sizes and are secured with one of four shims, allowing placement of the implant in several different positions. Specifically, the implant can be secured in a horizontal or vertical position, at the superior or inferior boarder of the thyroplasty window, and at any position along the anterior to posterior position of the thyroplasty window (Fig. 15.7). Fig. 15.7 The VoCoM implant and shim system for vocal cord medialization. There are several advantages inherent in the design of this system, which streamlines the actual surgical procedure. This is critical to the success of the thyroplasty technique, as tissue edema, which develops from excessive operative time or manipulation, can make it difficult for the surgeon to judge the appropriate vocal endpoint. A specially designed surgical instrument set facilitates window placement, determination of implant size and optimal location, and insertion of the implant. The graduated prefabricated implants and shims obviate the need to hand carve implants on the back table during the procedure, saving valuable operative time. The implant is made of hydroxylapatite with proven biocompatibility that generates a thin fibrous encapsulation. In some individuals there may be osteogenesis in the region of the fenestra, creating lamellar bone bridging between the implant and the thyroid lamina.13 For individuals with paresis and residual motion, this provides implant stability and minimizes the risk of migration or extrusion. Although the osteogenesis is localized and does not preclude implant removal, this system should not be used in individuals in whom removal is anticipated. The main disadvantage of the system is that the firm nature of the implant does not allow for further carving, and additional modification of the shape must be done with a diamond drill. Additionally, osteointegration in the area of the thyroid lamina may make the procedure less easily reversible than other implants such as Silastic. Technique A 4- to 5-cm horizontal incision is made over the lateral aspect of the thyroid lamina and extended 1 cm across the midline (Fig. 15.8). Subplatysmal flaps are elevated superiorly and inferiorly, and the strap muscles are separated in the midline and retracted laterally. Fibers of the thyrohyoid are divided with electrocautery to delineate the inferior boarder of the thyroid cartilage, and the thyroid lamina is exposed by retraction of the strap muscles laterally and by rotating the larynx to the contralateral side using a single hook placed at the thyroid notch. Fig. 15.8 Thyroplasty technique. (A) Skin incision. (B) After elevation of subplatysmal flaps, the strap muscles are divided and retracted laterally. (C) The larynx is rotated using a single hook, and the fenestra template tool is used to mark the location of the window. (D, E) The window can be fashioned using a scalpel, Kerrison punch, or drill. (F) The paraglottic tissues are freed from the inner table of the thyroid cartilage. (G) A series of trial inserts are placed to determine optimum implant size and position, after which the final implant will be placed and secured with the appropriate shim. The position of the vocal fold relative to external landmarks is shown. (H) The implants can be placed vertically or horizontally to achieve optimum phonation. (From Cummings CW, Haughey BH, Thomas JR, et al, eds. Otolaryngology: Head and Neck Surgery. 4th ed. St. Louis: Elsevier-Mosby, 2005:2194. Reprinted with permission of Elsevier. Copyright © 2005.) To prevent coughing during implant manipulation, topical anesthetic can be injected into the subglottic airway or 50 to 100 mg of lidocaine can be given intravenously just prior to the entry through the thyroid lamina. Interestingly, no local anesthetic is needed in the paraglottic tissues during implant manipulation. The position of the cartilage window is determined. The superior aspect of the window should be placed at the level of the true vocal fold. This position lies at the halfway vertical distance between the fundus of the thyroid notch and the anterior inferior edge of the thyroid cartilage. A line from this point extending posteriorly parallel to the inferior boarder of the thyroid cartilage will approximate the level of the true vocal fold (TVF). For females, the anterior aspect of the window is positioned 5 to 8 mm lateral to the midline and for males 8 to 10 mm. The window is carefully outlined using the template, and the outer perichondrium and cartilage are removed taking care to accurately maintain the dimensions of the window (this is important to ensure a snug fit of the prosthesis). The cartilage can be removed using a scalpel, a Kerrison punch, or a small otologic drill. If possible, the integrity of the inner perichondrium is preserved. Some experts place the implant external to the inner perichondrium, and others feel that the perichondrium tethers the medialization and strip it away. Regardless, care should be taken to ensure hemostasis and to make sure that the airway is not violated. The paraglottic tissues are carefully freed from the inner table of the thyroid cartilage using the perichondrial elevator. A series of trial implants are then placed ranging from 3 to 7 mm of displacement (Fig. 15.9). The implants can be rotated into four orientations and placed throughout the four quadrants of the window to determine the placement for optimal phonation (Fig. 15.10). We have found that the most common position is in the inferior posterior quadrant in the vertical position with the bevel facing inferiorly. To medialize the vocal process of the arytenoid, the implant can be rotated to the horizontal position and placed posteriorly. The patient is asked to vocalize to confirm optimal placement. The trial implant is then removed. Fig. 15.9 Trial implant tools alongside the corresponding final implants. Fig. 15.10 Depending on the shim used, the implant can be variably positioned along the anterior/posterior and superior/inferior axis of the fenestra. If inadequate voicing is obtained, and a persistent posterior glottic gap is evident on laryngoscopy, an arytenoid adduction procedure can be considered at this point, prior to placement of the final implant. Medialization thyroplasty does not affect the level of the vocal fold in the vertical plane. If there is significant discrepancy, it may be necessary to add an arytenoids repositioning procedure to the medialization. It is important that once the window is created to proceed with implant placement with alacrity to minimize distortion of the voice from glottic edema. The appropriate implant and shim are chosen. Just before placement of the final prosthesis, the field is flooded with saline and the patient is asked to perform a Valsalva maneuver. Any appearance of air bubbles suggests violation of the airway, in which case the procedure should be terminated. Assuming the integrity of the airway has been maintained, the implant is loaded onto the handle of the implant inserter and placed in the proper position. The correct shim is then placed using a smooth dressing forceps, thus securing the implant in the desired position in the fenestra (Fig. 15.11). The wound is irrigated with antibiotic solution. A drain is placed deep to the strap muscles. The strap muscles and platysma are closed with an absorbable suture and the skin is closed as desired. Fig. 15.11 An implant in situ just prior to closure. Postoperatively, the patient is monitored overnight in the hospital for possible hematoma formation and airway obstruction. The drain is removed on postoperative day 1. The patient is given a regular diet but encouraged to minimize aggressive vocal activity such as coughing, throat clearing, or yelling, although absolute voice rest is not necessary. Patients are told that the voice may deteriorate on the first postoperative day, but will reemerge between postoperative days 3 and 5. Prophylactic antibiotics are continued for 5 days.
Vocal Fold Medialization, Arytenoid Adduction, and Reinnervation
Laryngeal Framework Surgery
Isshiki’s Classification
Thyroplasty Type I
Thryoplasty Types II to IV
Arytenoid Adduction
Principles and Theory of Laryngeal Framework Surgery
Procedures
Netterville’s Technique for Silastic Implants
Meyer and Blitzer’s Rationale and Technique for Using the VoCoM (Nonporous Hydroxylapatite Ceramic) Prosthesis

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







