Axial T2-weighted MRI scan of a man with chronic toluene abuse and dementia. Arrows point out white matter hyperintensity, and there is also lateral ventricular enlargement (from Filley and Kleinschmidt-DeMasters, 2001).
The suspicion that brain white matter bore the brunt of injury related to toluene was quickly confirmed by a postmortem study of a long-term inhalant abuser who died as a result of toluene-induced cardiac arrhythmia (Rosenberg et al., 1988a). The autopsy demonstrated diffuse myelin pallor in the cerebral and cerebellar white matter, with perservation of neurons throughout the brain, consistent with selective toxic changes in myelin (Rosenberg et al., 1988a). Later studies showed that this case was typical of toluene abuse (Kornfeld et al., 1994; Fornazzari et al., 2003; Filley, Halliday, and Kleinschmidt-DeMasters, 2004; Al-Hajri and Del Bigio, 2010), and it became increasingly clear that myelin was the principal target of this agent, sparing cell bodies and even axons in all but the most severe cases. The large white matter tracts of the cerebrum and cerebellum were primarily affected, and in the cerebral hemispheres the periventricular white matter was most affected while subcortical U fibers were relatively spared (Rosenberg et al., 1988a; Filley et al., 2004). Of note, when the intracortical white matter was investigated, it was found to be either unaffected (Rosenberg et al., 1988a; Filley et al., 2004) or minimally involved (Kornfeld et al., 1994). Moreover, the importance of cerebral white matter involvement in the pathogenesis of dementia was made clear by a study of toluene abusers showing that the degree of cognitive impairment correlated not only with the duration of toluene abuse but with the severity of white matter change on MRI (Filley, Heaton, and Rosenberg, 1990). These observations were subsequently confirmed by many similar studies around the world (Yücel et al., 2008).
The chronic sequelae of toluene abuse therefore served to transform the understanding of dementia and its neuropathological basis. White matter was now unequivocally a consideration in the pathogenesis of dementia, and, even in its nascent form, this idea deserved formal designation. So it was that in 1988, based primarily on experience with this syndrome (Hormes, Filley, and Rosenberg, 1986; Filley, Heaton, and Rosenberg, 1990) but also on detailed study of MS (Franklin et al., 1989) and other disorders, the syndrome of WMD was proposed to describe the dementia that can accompany brain white matter involvement (Filley et al., 1988). Implicit in the idea was that white matter disorders typically involve widespread areas, and the disruption of myelinated tracts affects cognition regardless of the specific neuropathology present. Toluene leukoencephalopathy stands out today as the most convincing example of this syndrome, although both the novelty of the WMD concept and the relative invisibility of inhalant abuse continue to hinder the appreciation of this emerging area of behavioral neurology.
Definition and characterization
WMD is defined as a dementia syndrome resulting from diffuse or multifocal cerebral white matter damage (Filley et al., 1988; Filley, 1998; Schmahmann et al., 2008). Implicit in this definition is the assumption, based on experience with all the white matter disorders, that the total amount of white matter damaged determines the nature and severity of the cognitive impairment. That is, if dementia is destined to occur in a white matter disorder, a sufficient amount of cerebral white matter must be rendered dysfunctional. This assumption does not necessarily mean that white matter is equipotential in its mediation of higher functions. Instead, the WMD concept implies that a critical amount of tissue involved in multiple distributed neural networks must be affected before clinical features develop. Much as the extent of cortical disease is thought to accumulate to the point of clinical impairment in cortical dementias such as AD, so too does white matter disease advance in the stages leading to the syndrome of WMD. The notion of WMD should not be taken to imply that focal white matter lesions have no specific neurobehavioral correlates, as indeed a host of focal syndromes are well known to be associated with discrete white matter lesions (Geschwind, 1965; Filley, 2012). Rather, WMD develops when a sufficient lesion burden is present across several parallel networks to reach the point where dementia ensues. As the idea matured, it became clear that all recognized white matter disorders have the potential to produce a similar profile of cognitive impairment. Because these disorders tend to be multifocal or diffuse in their distribution, it was appreciated that multiple tracts are typically affected, and a neurobehavioral profile of multitract involvement began to emerge. The realization of this synthesis was nothing less than astonishing, as an impressive range of seemingly disparate disorders all became united by virtue of their common origin in white matter.
Overview
The clinical profile of WMD has been investigated by considering the wide variety of white matter disorders as a group. Box 7.1 displays the profile of WMD as currently understood, including expected cognitive deficits and areas of preserved function. This profile resembles other, more familiar characterizations of dementia (McKhann et al., 1984; Mendez and Cummings, 2003; American Psychiatric Association, 2013), but emphasizes features that are uniquely relevant to dementia associated with white matter disorders.
Cognitive slowing
Executive dysfunction
Sustained attention impairment
Memory retrieval deficit
Visuospatial impairment
Psychiatric disturbance
Relatively preserved language
Normal extrapyramidal function
Normal procedural memory
In the years after the WMD proposal in 1988, further investigation steadily shed light on the hypothesis (Filley et al., 1989; Merriam, Hegarty, and Miller, 1989, 1990; Filley et al., 1990; Filley and Gross, 1992; Swirsky-Sacchetti et al., 1992; Rao et al., 1993; Filley and Cullum, 1994; Shapiro et al., 1994; Yamanouchi et al., 1997; Filley et al., 1999; Riva, Bova, and Bruzzone, 2000; Mendez, Perryman, and Bronstein, 2000; Harris and Filley, 2001; Price et al., 2005; Lafosse et al., 2007; Shibata et al., 2007; Libon et al., 2008; Filley et al., 2009; Grigsby et al., 2014), and several reviews have dealt with conceptual issues (Rao, 1993, 1996; Filley and Kleinschmidt-DeMasters 2001; Feinstein, 2007; Schmahmann et al., 2008; Coltman et al., 2011; Al-Hasani and Smith, 2011; Miyamoto et al., 2013; Kloppenborg et al., 2014; Prins and Scheltens, 2015). Systematic study of the distinctions between these dementia categories has been undertaken to a considerable extent (Filley et al., 1989; Gallassi et al., 1991; Rao et al., 1993; Derix, 1994; Bennett et al., 1994; Doody et al., 1998; Iddon et al., 1999; Aharon-Peretz, Kliot, and Tomer, 2000; Lafosse et al., 2007; Lafosse et al., 2013). These and many other contributions have contributed to ongoing refinement of the idea.
A crucial objective is to distinguish WMD from both cortical and subcortical dementia, and studies comparing MS with AD (Filley et al., 1989) and MS with Huntington’s Disease (HD) (Lafosse et al., 2007) were instructive. First, in comparing the dementia of MS with AD – diseases of white and cortical gray matter – it was shown that while MS features processing speed and executive dysfunction, AD manifests memory encoding and language deficits (Filley et al., 1989). Then, comparison of MS with HD – diseases of white and subcortical gray matter – disclosed that while both diseases produce attentional and memory retrieval deficits, MS does not affect procedural memory whereas HD is associated with impairment in this domain (Lafosse et al., 2007). These reports were foundational in establishing that WMD differs from both cortical and subcortical gray matter dementia. WMD was distinguished from cortical dementia by relative normalcy of language and declarative memory encoding while cognitive speed, executive function, and sustained attention are impaired, and from subcortical dementia by sparing of procedural memory and extrapyramidal function (Filley et al., 1988; Filley, 1998, 2011). Core distinctions between the three syndromes are most specifically apparent in aspects of memory and language, differences that are displayed in Table 7.1.
| Domain | Cortical dementia | White matter dementia | Subcortical dementia |
|---|---|---|---|
| Declarative memory | Amnesia | Retrieval deficit | Retrieval deficit |
| Procedural memory | Normal | Normal | Impaired |
| Language | Aphasia | Normal | Normal |
As time progressed and new data emerged, the importance of cognitive slowing also became apparent. Whereas slowed cognition was long associated with the subcortical dementias (Albert et al., 1974; McHugh and Folstein, 1975), and indeed all dementia patients may struggle with slowed processing speed, the advent of advanced neuroimaging allowed detailed study of white matter tracts in vivo to address this issue specifically. When the white matter is specifically queried by diffusion tensor imaging (DTI), for example, cognitive processing speed has been tightly linked with the integrity of myelinated systems (Turken et al., 2008; Penke et al., 2010, Haász et al., 2013). Disorders that impair central impulse conduction thus produce slowed cognition – a reasonable assumption now increasingly supported by neuroimaging evidence – and cognitive slowing has become a distinctive feature of WMD. Thus a patient with entirely normal gray matter of the cerebral cortex and deep nuclei may still experience slowing of cognition from damage to white matter tracts alone. With this important addition to the list of cognitive deficits and areas of relative strength, the clinical profile of WMD now highlights this deficit (Box 7.1).
A clinically helpful point deserving emphasis is the tendency for the differentiating characteristics of dementias to be most evident in the early and middle stages of the disorder. Thus, although typical cognitive deficits associated with diffuse white matter lesions may progress over time (Kloppenborg et al., 2014; Smith et al., 2015), WMD is more easily detected before it becomes severe. This feature is particularly useful in that it assists in the diagnosis of dementia when the greatest opportunity for effective treatment still presents itself. As the dementia progresses, the accumulation of neuropathology usually results in a clinical picture of neurobehavioral and neurologic disability that appears increasingly uniform. In the most severe and terminal stages, little if any distinction between cortical, subcortical gray matter, or white matter dementia can be detected. In short, as neuronal loss in the brain from any cause proceeds, all dementias increasingly come to resemble one another. However, the early stages of dementia manifest important differences, as will now be discussed in more detail.
Cognitive slowing
Among the most obvious deficits that might be expected in patients with white matter disorders is cognitive slowing, often known alternatively as slowed speed of information processing or psychomotor slowing. As discussed in Chapter 3, the core neurophysiological function of myelin is the enhancement of axonal conduction velocity, and all white matter disorders discussed in this book share a prominent disturbance of this process. The idea that white matter disorders induce cognitive slowing is disarming in its simplicity, but this clinical phenomenon quite accurately reflects the neurobiology involved. In patient populations as well as normal subjects, recent MRI and DTI observations have shown that the integrity of white matter tracts significantly impacts the speed of cognitive processing (Turken et al., 2008; Bartzokis et al., 2008; Kochunov et al., 2010; Penke et al., 2010; Bendlin et al., 2010; Haász et al., 2013). Thus it is appropriate to conclude that, generally stated, neurons that are slow to conduct impulses contribute to slowed thinking. Other conditions that exert their impact on gray matter may of course also produce slowed cognition, but the primacy of cognitive slowing seems particularly characteristic of white matter disorders.
Cognitive slowing has been emphasized as a cardinal feature of subcortical dementia (Cummings, 1990), and the central role of white matter in frontal-subcortical networks implies that impaired timing and activation of cortical systems can result from diffuse white matter involvement. Slowed information processing has been correlated with vascular white matter disease (Kloppenborg et al., 2014), and has long been observed in MS (Litvan et al., 1988; Demaree et al., 1999), allying white matter disorders with the subcortical gray matter diseases broadly considered. Impaired cognitive speed will most often be apparent in everyday tasks dependent on rapid information processing, and, as a result, patients may complain of being overwhelmed with the burden of multiple tasks present in a work setting. In the clinical encounter, cognitive slowing contributes most importantly to impairments in attention, and memory retrieval is also affected, as will be discussed later. A close relationship exists, for example, between cognitive speed and sustained attention (Weinstein et al., 1999). Evidence can be found to suggest that “cognitive fatigue” contributes to poor performance on tasks requiring sustained attention such as the Paced Auditory Serial Addition Test (PASAT; Krupp and Elkins, 2000; Schwid et al., 2003). In the neuropsychology laboratory, this deficit will be evident on vigilance tests, and an increase in reaction time may coexist with a deficit in sustained attention (Rueckert and Grafman, 1996). Indeed, some have suggested that impairments in vigilance are entirely explained by cognitive slowing (Spikman, van Zomeren, and Deelman, 1996). However its specific mechanisms are delineated, cognitive slowing is a common impairment that often dominates the behavioral repertoire of patients with white matter disorders of any type. In the context of WMD, cognitive slowing is best viewed as a deficit that most obviously becomes manifest in the performance of attentional, memory, and executive function tasks.
Executive dysfunction
The capacity to plan, carry out, monitor, and complete cognitive tasks while avoiding distraction is the essence of what has come to be regarded as executive function. This domain is crucial to normal cognition, and its dissolution can be observed in many brain diseases. Executive dysfunction has steadily risen to the status of a defining deficit in the white matter disorders affecting cognition (Filley, 2010). White matter involvement in Binswanger’s Disease (BD), for example, prominently impairs executive dysfunction (Román et al., 1993), and a severe burden of ischemic white matter disease produces selective executive deficits that are not explained by cortical amyloid deposition (Yoon et al., 2013).
Executive dysfunction is a feature of toxic leukoencephalopathies related to toluene and other agents, and results primarily from frontal lobe myelin loss (Filley and Kleinschmidt-DeMasters, 2001; Filley, Halliday, and Kleinschmidt-DeMasters, 2004). Deficits on tests that measure executive function have similarly been noted in MS patients (Feinstein, 2007), and MRI studies have correlated these deficits with demyelinative plaques in the frontal white matter (Arnett et al., 1994). In comparing patients with normal pressure hydrocephalus (NPH) to those with AD, Iddon and colleagues (1999) found that a pattern of executive dysfunction related to frontal lobe involvement distinguished the former from the latter. In patients with metachromatic leukodystrophy (MLD), a frontal lobe syndrome with impaired executive function has been documented neuropsychologically (Shapiro et al., 1994), and executive dysfunction is the most significantly affected cognitive domain in fragile X tremor ataxia syndrome (FXTAS; Grigsby et al., 2014).
Sustained attention impairment
Attention is a difficult and multifaceted concept in behavioral neurology and neuropsychology. While useful in that it designates a set of important mental operations, the meaning of attention depends on the context in which it is used. In the most general sense, attention refers to the ability to focus on some stimuli while competing distractors are present; this capacity, often called selective attention, operates over a period of seconds, and is usefully tested by the digit span (Mesulam, 2000). When selective attention operates over a period of minutes, sustained attention, also known as concentration or vigilance, is engaged (Filley and Cullum, 1994), and a variety of continuous performance tasks are suitable for assessment of this task (Mesulam, 2000). Sustained attention is also closely related to the notion of working memory, and similar brain regions appear to be involved in each.
In a study testing the idea that sustained attentional disturbances are prominent in white matter disorders, a comparison of MS and AD patients disclosed that sustained attention was markedly affected in the former while relatively normal in the latter (Filley et al., 1989). This study was the first to compare white matter and cortical disease directly from a neurobehavioral perspective, and demonstrated contrasting profiles of attentional and concentration dysfunction in patients with MS versus memory and language impairment in those with AD; from these observations it was proposed that these two diseases represent prototype white matter and cortical dementias (Filley et al., 1989). Attention and concentration deficits were later found to be more common in BD than in AD (Doody et al., 1998), further supporting this distinction. These results are usefully interpreted in light of a neuropsychological comparison of subcortical gray with subcortical white matter disease (Caine et al., 1986); in this study, patients with MS and HD had similar cognitive impairment, but memory dysfunction was more severe in HD, and MS patients showed normal cognitive strategies but lowered mental efficiency. Thus in these respects MS differs from both AD and HD. Sustained attention can also be shown to decline in normal aging (Filley and Cullum, 1994), which not incidentally features a decline in white matter integrity (Bartzokis et al., 2010).
The neuroanatomy of sustained attention is not fully understood, although it is likely that a network of interconnected cerebral structures mediates this capacity. Many lines of evidence implicate the frontal lobes and their connections to more posterior regions (Mesulam, 2000), and some evidence suggests that the right frontal lobe is particularly specialized for sustained attention (Rueckert and Grafman, 1996). It also appears that frontal lobe white matter in particular contributes to this capacity (Filley, 2012).
A study of children with attention-deficit disorder with hyperactivity, for example, found a smaller volume of the right frontal lobe white matter than in normal controls; furthermore, poorer performance on sustained attention tasks was associated with reduced right hemisphere white matter volume (Semrud-Clikeman et al., 2000). More recently, DTI studies in normal children (Karlborg et al., 2013) and adults (Takahashi et al., 2010) have correlated the integrity of right frontal white matter with performance on sustained attention tasks. Evidence also exists for a role of the corpus callosum in sustained attention, as studies of children using tachistoscopic tasks have suggested that interhemispheric transfer of information is important for performance on tests of vigilance (Rueckert, Sorenson, and Levy, 1994; Rueckert et al., 1999).
Consistent with these observations, patients with acquired lesions of the corpus callosum have deficits in sustained attention. In MS, for example, an impairment of vigilance has been correlated with reduced corpus callosum size as assessed by MRI (Rao et al., 1989b). Similar reductions in the size of the corpus callosum have been reported in children with attention-deficit/hyperactivity disorder (Giedd et al., 1994). A considerable body of experimental evidence acquired from normal subjects also supports a prominent role for the corpus callosum in attentional processing (Banich, 1998).
Memory retrieval deficit
Memory, like attention, is a multifaceted and fundamental concept in clinical neuroscience. Many different varieties of memory have been postulated, derived from clinical examples in which specific deficits in memory can follow documented brain lesions (Budson and Price, 2005). For our purposes, two distinctions will prove most helpful. The first is the distinction between declarative and procedural memory. Declarative memory, routinely tested in neurologic encounters, is a mainstay of the mental status examination; two subtypes are episodic (events of personal experience) and semantic (conceptual and factual knowledge). Procedural memory, in contrast, refers to the learning of skills at an unconscious, automatic level, and this form of memory must be evaluated by special neuropsychological tests tapping the capacity for motor learning.
The second distinction involves the related but separable processes of encoding versus retrieval within declarative memory (Cummings, 1990). An encoding deficit, also known as amnesia, is widely thought to indicate hippocampal damage, and among the dementias, AD is by far the most common cause. A retrieval deficit, in contrast, implies that the information is encoded but cannot be readily accessed, and this kind of memory failure implicates extrahippocampal brain regions. The clinician has some capacity to evaluate these aspects of memory by determining if the recall of items that cannot be recalled after a short delay can be improved by one of two methods: (1) cuing the patient with the provision of semantic clues that may trigger a memory, and (2) providing a list of items in which the desired ones are included. If either of these procedures results in improved recall, the patient can be said to have encoded the information but been deficient in its retrieval. The examiner has thus documented preservation of recognition memory, and the patient can be considered to have a retrieval deficit. In the practice of behavioral neurology, this distinction can at times be established during the office or bedside evaluation; but neuropsychological testing can offer a more thorough assessment with standardized measures capturing more of the subtlety of memory impairment. The data on memory retrieval have been primarily derived from this approach, and in this light, it is pertinent to review some neuropsychological studies of memory that have suggested a unique pattern in patients with white matter disorders. The retrieval deficit will first be considered, and then normal procedural memory in a subsequent section.
Early efforts to define the type of declarative memory impairment in white matter disorders were undertaken with MS patients, and a retrieval deficit was found (Rao et al., 1984). As reviewed in Chapter 6, some studies have presented data supporting an alternative idea that the declarative memory deficit of MS patients is more related to difficulty with the acquisition of information than its retrieval (DeLuca, Barbieri-Berger, and Johnson, 1994; DeLuca et al., 1998). It may be that both processes are relevant, given the complex and time-dependent neuropathology of MS (Lafosse et al., 2013). Encoding deficits in MS, for example, may reflect demyelination in the alveus, an intrahippocampal white matter structure (Laule et al., 2008), as well as larger afferent and efferent hippocampal tracts. As gray matter involvement is minimal early in the course of MS (Hauser and Oksenberg, 2006), the initial memory deficits are most likely to be in retrieval.
Similar retrieval deficits have also been documented in ischemic vascular dementia (Lafosse et al., 1997; Libon et al., 1998; Reed et al., 2000); in radiation leukoencephalopathy (Armstrong et al., 2000; Armstrong, Stern, and Corn, 2001); in carbon monoxide intoxication (Chang et al., 2009); in TBI (Timmerman and Brouwer, 1999); in the AIDS dementia complex (ADC; White et al., 1997; Jones and Tranel, 1991); in CADASIL (Chabriat et al., 2009); and in fragile X premutation carriers (Hippolyte et al., 2014), indicating that this pattern of declarative memory loss may be applicable to many other white matter disorders.
Although subtle, these distinctions support the idea that white matter disorders disturb declarative memory in a specific and reproducible fashion. This pattern relates to the selective involvement of white matter tracts that produces disruption of memory retrieval more than encoding. The specific tracts involved have yet to be securely identified, but some information on this issue is available.
On the basis of clinical reports and functional neuroimaging data, Markowitsch (1995) suggested that the uncinate fasciculus is responsible for memory retrieval. Memory retrieval requires the engagement of working memory systems in the frontal lobes to enable the recall of information stored in the temporal lobes, and the tract connecting these regions is the uncinate fasciculus (Markowitsch, 1995). Support for this schema has recently come from functional MRI (fMRI) studies of memory retrieval in normal adults demonstrating a ventral frontotemporal network within which the uncinate fasciculus is situated (Barredo, Oztekin, and Badre, 2015). These data may indicate a specific cognitive role for a white matter tract that has not heretofore been understood to have a firm neurobehavioral affiliation, and invited further study with modern neuroimaging technology applied to a variety of white matter disorders in which memory is affected.
Taking advantage of these technologies, studies of MS (Sepulcre et al., 2008), carbon monoxide intoxication (Chang et al., 2009), and glioma (Papagno et al., 2011) have shown that a variety of frontotemporal tracts, including but not limited to the uncinate fasciculus, participate in memory retrieval. Consistent with this idea, DTI studies of normal elders have demonstrated that decline in the integrity of prefrontal white matter and the genu of the corpus callosum contribute to age-related slowing of memory retrieval (Bucur et al., 2008). As attractive as it may be to assign memory retrieval to one tract, a more complicated system appears to be operative, and a network of white matter structures likely participates in the retrieval of declarative memory (Sepulcre et al., 2008).
As in the case of attentional dysfunction, the retrieval deficit seen in individuals with white matter disorders can be seen as closely related to cognitive slowing. In clinical practice, these patients will often be noted to produce the correct answer to a question if sufficient time is provided, implying that the information is encoded but not easily retrieved. Thus the delay in providing the correct answer may be interpreted as slowed cognition rather than a memory deficit. In other words, the patient is indeed cognitively slow, but the reason for this slowing is a failure of memory retrieval. As intimated earlier, cognitive slowing, while important as a general observation, can be analyzed in more detail and interpreted in terms of cognitive dysfunction. The delineation of specific deficits, most obviously in attention and memory, that contribute to cognitive slowing is an important neuropsychological issue deserving further study.
Visuospatial impairment
Visuospatial function has only lately received attention, but studies that are available suggest an impairment related to white matter dysfunction. Studies on the genetics of normal fiber architecture have shown that the integrity of white matter is associated with full-scale IQ (FSIQ) and performance IQ (PIQ) but not verbal IQ (VIQ) (Chiang et al., 2009). These data indicate a selective relationship of white matter with nonverbal capacities that is in part genetically mediated. Given that diffuse white matter lesions are not strongly associated with language dysfunction, it might be predicted that white matter disorders have more prominent nonverbal manifestations, and this prediction has in fact been upheld.
To begin, focal lesion data clearly support a role of white matter in visuospatial dysfunction. A CT study of 53 patients with visuospatial dysfunction after right hemisphere stroke found that left neglect was highly correlated with damage in the temporoparietal white matter (Samuelsson et al., 1997). Left neglect has also been documented in an MS patient with a right cerebral demyelinative lesion (Graff-Radford and Rizzo, 1987). With respect to the literature on diffuse lesions, cross-sectional and longitudinal studies of diffuse white matter hyperintensities of vascular origin have disclosed that these lesions correlate with perceptual and constructional deficits (Kloppenborg et al., 2014). Similarly, MS patients with diffuse demyelinative lesions score about 10 points lower on the performance subtests of the Wechsler Adult Intelligence Scale than on the verbal subtests (Rao, 1996), and specific visuospatial deficits have been shown on a variety of standard tests of right hemisphere function (Heaton et al., 1985; Rao et al., 1991). Studies of solvent-induced leukoencephalopathy have confirmed that nonverbal abilities are more impaired than verbal skills in patients with toluene dementia (Yamanouchi et al., 1997). This nonverbal-verbal neuropsychological discrepancy has also been observed in children with hydrocephalus (Mataró et al., 2001) and in patients with metachromatic leukodystrophy (MLD; Shapiro et al., 1994). Using DTI, studies in normal young adults have found that visuospatial attention correlates with the integrity of white matter of the right parietal and temporal lobes (Tuch et al., 2005).
With respect to disease states, abstinent alcoholics were then shown to have impaired visuospatial function associated with DTI microstructural white matter involvement in bilateral frontal and temporal regions (Rosenbloom et al., 2009). Most recently, a DTI study of stroke patients correlated microstructural injury in right frontoparietal white matter with chronic left neglect (Lunven et al., 2015).
Psychiatric disturbance
Emotional and personality aspects of WMD have received considerable attention, and psychiatric syndromes have a complex relationship to white matter dysfunction (Filley, 2012). This area of neuropsychiatry is vast and poorly understood, and only a brief account can be offered here; comprehensive reviews have recently addressed intriguing new ideas on the relationship of white matter to psychiatric illness in general (Bartzokis, 2011; Walterfang et al., 2011; Haroutunian et al., 2014). For the purposes of this book, some of the most important observations have been made in patients with known neurologic diseases. Psychosis as an early feature of adult-onset MLD, for example, was noted as a frequent trend (Filley and Gross, 1992; Hyde, Ziegler, and Weinberger, 1992), and the development of this syndrome was interpreted as an early component of a sequential progression to dementia seen in these patients (Filley and Gross, 1992; Shapiro et al., 1994).
Depression in MS has received much attention (Minden and Schiffer, 1990; Feinstein, 2007), and the potential lethality of this problem was better appreciated as a high risk for suicide was documented (Sadovnick et al., 1991). It is likely that depression in MS results at least in part from frontotemporal white matter disease, and exacerbates cognitive dysfunction related to other brain involvement (Feinstein, 2007).
Depression has been reported to be more common in BD patients than in AD patients who are comparably demented (Bennett et al., 1994). In white matter lacunar dementia, the severity of delusions and hallucinations, aggression, irritability, aberrant motor behavior, nighttime behavior, and appetite changes has been correlated with cognitive decline, whereas no such correlations were found in AD (Aharon-Peretz, Kliot, and Tomer, 2000). These data suggest that white matter ischemia and infarcts have a direct impact on psychosis and related syndromes.
A provocative recent contribution to this area has been the proposal that white matter immaturity may predispose to psychiatric disease in adolescence and young adulthood. Adopting a developmental perspective, Bartzokis (2005) suggested that the process of myelination in adolescents and young adults is incomplete, possibly contributing to the high incidence of psychiatric conditions such as schizophrenia, mood disorder, attention-deficit/hyperactivity disorder, and conduct disorder at this time of life. These disorders all tend to reduce the already tenuous inhibitory control of adolescence, thus predisposing to the higher incidence of addiction, the effects of which may further inhibit white matter maturation (Bartzokis, 2005).
Relatively preserved language
One of the most robust observations in the white matter disorders is that language is usually well preserved. In this respect, the classical teachings of clinical neurology are entirely accurate. Genetic studies failing to detect a link between normal white matter and verbal function as measured by VIQ (Chiang et al., 2009) have been mentioned earlier. Among clinical populations, aphasia is uncommon in the setting of white matter disease (Derix, 1994; Filley, 2012), and language impairment, if present, is typically subtle. In the investigation of vascular white matter disease, language has not often been evaluated (Kloppenborg et al., 2014), but cross-sectional studies have not found a correlation between the extent of white matter hyperintensities and language dysfunction (Murray et al., 2010; Price et al., 2012).
In people with MS, aphasia is indeed rare, and a recent large multicenter study found that less than 1% of MS patients experience this syndrome (Lacour et al., 2004). When it does occur, aphasia takes reasonably predictable forms. As expected from the classic model of aphasia localization (Filley, 2011), for example, conduction aphasia can appear in relation to a focal plaque in the left arcuate fasciculus (Arnett et al., 1996). With detailed testing, minor deficits in language can be detected in MS (Kujala, Portin, and Ruutianen, 1996), but these are typically not evident in ordinary discourse or even on routine mental status testing. In comparison to patients with AD, those with MS have little linguistic difficulty (Filley et al., 1989).
Impaired verbal fluency may be seen in some white matter disorders (Derix, 1994, Filley, 2010), reflecting executive dysfunction at least as much as linguistic disturbance. Speech disorders, however, are frequent in white matter disorders. Dysarthria is well known in MS, and can sometimes assume a scanning quality. Articulation deficits are also described in the acquired immunodeficiency syndrome (AIDS; Navia, Jordan, and Price, 1986), toluene leukoencephalopathy (Hormes, Filley, and Rosenberg, 1986), and BD (Babikian and Ropper, 1987). In these disorders, involvement of corticobulbar tracts subserving articulation is plausible.
Despite the fact that language is affected only mildly in WMD, or not at all, it should not be overlooked that white matter still has a prominent role in language. From the stroke literature, for example, it is indeed clear that left perisylvian white matter involvement plays a role in all traditional aphasia syndromes (Alexander, Naeser, and Palumbo, 1987). The resolution of this paradox is that language is highly lateralized and localized, and thus less affected by the diffuse neuropathological distribution of typical white matter disorders. Language is vulnerable to large, focal left perisylvian lesions, most often of vascular origin, but is not typically implicated in WMD, which results from less dramatic but more widely distributed injury to a broad array of tracts elsewhere in the brain. The cortical regions responsible for language processing – Broca’s area, Wernicke’s area, and adjacent cortices – are interconnected by heavily myelinated tracts that extend relatively short distances (Anderson, Southern, and Powers, 1999) and are therefore less susceptible to most white matter neuropathology. Thus, whereas large left hemisphere infarcts damaging language-related white matter tracts can surely produce aphasia (Alexander, Naeser, and Palumbo, 1987), the diffuse white matter lesions implicated in WMD do not usually lead to aphasia because they mainly affect nonlinguistic tracts, or are of insufficient size to exert a notable effect on language.
Normal extrapyramidal function
Extrapyramidal dysfunction – in the form of tremor, dystonia, chorea, athetosis, tic, myoclonus, and so forth – is traditionally associated with disorders of the subcortical gray matter, and neurologists who detect these signs in the evaluation of dementia patients are usually correct in assuming that white matter disorders are not typically responsible. Despite this useful clinical adage, an initial criticism of the WMD hypothesis questioned the usefulness of the absence of movement disorders based on the observation that these phenomena may be encountered in white matter diseases (Merriam, Hegarty, and Miller, 1990). However, it is well accepted that movement disorders are typically caused by basal ganglia neuropathology, often combined with thalamic and brain stem involvement (Jellinger, 1998), and white matter disorders do so only when myelinated tracts within the deep gray matter are implicated. A large study of older patients seen in memory clinics did in fact detect extrapyramidal signs in 10%, but these were not strongly related to the burden of white matter hyperintensity on MRI (Staekenborg et al., 2010).
Movement disorders reflecting basal ganglia involvement can occasionally occur in white matter disorders that have reached a late stage, as in the case of myoclonus in the AIDS dementia complex (Navia, Jordan, and Price, 1986), or when the leukoencephalopathy is sufficiently widespread to involve the white matter of the basal ganglia, as exemplified by parkinsonism from severe carbon monoxide poisoning (Sohn et al., 2000). In MS, movement disorders are very rare (Ozturk et al., 2002), and some may actually be coincidental to demyelinative disease (Tranchant, Bhatia, and Marsden, 1995).
As few rules in behavioral neurology are absolute, extrapyramidal dysfunction can be seen in white matter disorders, but these cases typically can be explained by some degree of subcortical nuclear involvement, often in the later stages of a white matter disorder, or by an unrelated movement disorder. In the clinic, it remains justified, as a general rule, to associate movement disorders with subcortical gray matter diseases (Ropper, Samuels, and Klein, 2014).
Normal procedural memory
Procedural memory, along with its companion procedural learning, represents an area of cognitive neuroscience that is only peripherally related to clinical neurology and neuropsychology. Patients rarely complain of dysfunction in the performance of previously acquired or newly sought motor skills, and if they do, issues such as joint disease, fatigue, and forgetfulness are often invoked as explanatory. Moreover, procedural memory is challenging to assess and cannot be routinely attempted in a clinical setting. Yet procedural memory is an important cognitive capacity and has relatively secure neuroanatomic correlates.
In WMD, procedural memory has been particularly informative. Studies of MS have found a preservation of procedural memory (Rao et al., 1993), as have investigations of the ADC (White et al., 1997; Jones and Tranel, 1991) and TBI (Timmerman and Brouwer, 1999). Experiments with laboratory mice have demonstrated that recall of a prelearned motor skill does not require active myelination (McKenzie et al., 2014), implying that procedural memory can be preserved as normal in humans with white matter disorders. Procedural memory is also relatively preserved in AD (Grafman et al., 1990). In contrast, HD patients show impaired procedural memory (Knopman and Nissen, 1991; Gabrieli et al., 1997). These studies suggest that diseases affecting the basal ganglia are most likely to disrupt procedural memory.
To assess the specific role of white matter in procedural memory, Lafosse and colleagues (2007) compared clinically definite MS patients, genetically verified HD patients, and normal controls, and found that, as hypothesized, MS patients and HD patients share a retrieval deficit in declarative memory, but MS patients have normal procedural memory, as tested by rotary pursuit, while HD patients are impaired (Figure 7.2). The findings with respect to spared procedural memory in MS support the notion that procedural memory dysfunction may distinguish white matter disorders from the subcortical gray matter diseases (Lafosse et al., 2007). These neuropsychological results thus generally support a specific pattern of memory loss proposed for WMD, contrasting with both cortical disease, in which there is an encoding deficit in declarative memory and normal procedural memory, and subcortical gray matter diseases, in which there is a retrieval deficit and impairment of procedural memory (Filley, 1998, 2010).
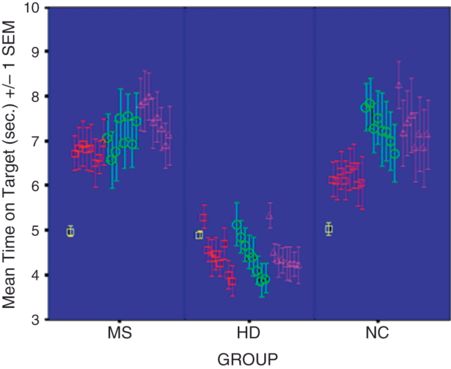
Rotary pursuit performance in patients with MS and HD compared to control subjects. HD patients, but not those with MS, show impaired procedural learning (from Lafosse et al., 2007).
To help conceptualize the essence of WMD, a crucial distinction should be maintained between the core functions of white matter – information transfer – and those of gray matter – information processing (Filley, 2010). White matter is characterized by extensive macroconnectivity, which integrates neuroanatomically distant functionally connected gray matter regions into coherent neural networks, and gray matter is characterized by massive microconnectivity, referring to the extensive synaptic networks by which individual neurons communicate with each other in the processing of information. Both constituents of the brain are necessary for normal behavior. WMD implies that distributed neural networks subserving cognition are less well organized, not as efficient, and ill-suited to integrate the impressive panoply of seamless cognitive operations implied by the concept of information processing (Filley, 2010). Whereas dementia of any type may proceed clinically to the same terminal outcome, the brain–behavior relationships involved in the onset, pathogenesis, and course of WMD are distinctive.
Unresolved issues
Any new proposal such as WMD entails a host of issues that require continued study and resolution. Just as the dichotomy of cortical and subcortical dementia engendered considerable resistance after its promotion in the 1970s and 1980s, so too may WMD seem foreign to those who regard the cortex as the unique repository of cognition. Whereas no one would contend that a patient deprived of all brain white matter by some neuropathological insult would have normal cognition, the precise role of white matter has yet to be fully established. Given the complex structural relationships of white and gray matter, a nuanced approach will be most productive. In other words, there may be relatively few patients in whom pure WMD – or for that matter cortical or subcortical dementia – appears, but these constructs nonetheless organize thinking so that the dynamic interplay of all regions can be considered in furthering the understanding of brain–behavior relationships relevant to dementia.
One important issue derives from the observation that some patients with extensive white matter hyperintensity on MRI may escape any cognitive disturbance (Fein et al., 1990). Examples such as these present a perplexing counterargument that challenges the idea of WMD, but may plausibly be explained by the concept of reserve, or the capacity of an individual to enjoy reduced vulnerability to dementing brain diseases because of premorbid protective factors. Reserve can be of two kinds: brain reserve – in essence a bigger brain with more neurons – or cognitive reserve – a brain with more synapses as a consequence of educational or occupational attainment before disease onset; but the demarcation of these categories is not absolute (Stern, 2009). In cortical dementia such as AD, a protective effect is likely mediated by increased synaptic density within cortical gray matter that counteracts the effects of neuritic plaques and neurofibrillary tangles, and a similar principle may apply to white matter disorders. For example, in neuropsychological studies of both adults with MS (Sumowski et al., 2010) and normal elders (Brickman et al., 2009), all of whom had some MRI white matter lesions, cognitive reserve was found to mitigate the impact of white matter pathology. Thus the highly educated, intellectually active, and socially engaged individual may be able to lessen or even avoid the cognitive impact of white matter lesions because of well-prepared cortical systems in which rich synaptic density can compensate for the specific effects of the newly acquired pathology. In this regard, a recent combined functional MRI and DTI study of normal elders found that performance on executive function and memory tasks was associated with both higher cortical activity and diminished white matter integrity, consistent with the notion of “less wiring, more firing” as a consequence of age-related changes in white matter (Daselaar et al., 2015). Further study of the idea of reserve with respect to the dementias will be of considerable interest.
A major concern with the concept of WMD is the issue of coexistent gray matter neuropathology. One of the most appropriate questions in this work is the degree to which concurrent gray matter involvement may explain the cognitive deficits. Discussion of this issue begins with the accumulated weight of correlational evidence justifying the existence of WMD in many patients with many disorders (Filley, 2012). Toluene leukoencephalopathy remains the most convincing cause of WMD, with confirmatory neuroimaging, neuropsychological, and neuropathological evidence (Yücel et al., 2008; Al-Hajri and Del Bigio, 2010) continuing to support the earlier reports making this claim (Hormes, Filley, and Rosenberg, 1986; Rosenberg et al., 1988b; Filley et al., 1990; Filley et al., 2004). In this disorder, brain myelin appears to be diffusely damaged by the highly lipophilic solvent toluene, leading to dementia that cannot be plausibly attributed to cortical neuronal injury, synaptic loss, or deep gray matter damage.
However, toluene leukoencephalopathy is a unique example of white matter toxicity, and few cognitive disorders can be attributed to exclusive white matter involvement. As discussed in Chapter 5, the relative contributions of white and gray matter changes may both be relevant. It is therefore essential that future studies combine state-of-the-art neuroimaging of both white and gray matter regions with detailed cognitive evaluation to address this issue. An example of such work can be found in an innovative study of Mendez and colleagues (2000) comparing 28 patients with dementia from various causes who had 25% or more of the subcortical white matter affected on conventional MRI with 28 AD patients; the former group had greater difficulty with cognitive speed and sustained attention, but better recognition memory, consistent with the profile of WMD.
Another issue with the concept of WMD has been the lack of a suitable animal model for laboratory study. Experiments with nonhuman animals are indeed hindered by limitations inherent in the use of such animals to investigate human brain–behavior relationships. Nevertheless, some support for the WMD idea is available because some investigators have succeeded in examining selective white matter pathology. The work of Gennarelli and colleagues (1982) on TBI in monkeys, for example, showed that the degree of diffuse axonal injury (DAI) was directly proportional to the length of coma and the quality of outcome. These experimental injuries were very severe, however, and in light of recent human studies suggesting that mild but repetitive TBI may have similar effects on white matter, repetitive mild TBI has been examined in mice; results disclosed ongoing memory impairments and white matter degeneration for up to 12 months after injury (Mouzon et al., 2014). Another useful category of neuropathology that can be studied experimentally is vascular disease. In mouse models of cerebral ischemia (Shibata et al., 2007; Coltman et al., 2011; Miyamoto et al., 2013), bilateral carotid artery stenosis damaging only the brain white matter has been shown to produce selective working memory impairment. Further experiments such as these are likely to add much to our knowledge.
Leaving aside for a moment the intricate details of how much white matter versus gray matter may be implicated in a given neurobehavioral syndrome, it is pertinent to reflect on the corticocentric bias that so dominates current neuroscientific thinking. If a patient has a cognitive deficit syndrome related to a focal cerebral lesion affecting both cortex and white matter, the explanation offered by most neurologists is that the cortical component of the lesion must be the critical area of damage. A left hemisphere stroke causing aphasia, for example, may often involve both cortex and white matter, but the gray matter portion is deemed crucial while the white matter damage is ignored. Such an opinion may be premature, as the contribution of white matter to language has been well demonstrated (Alexander, Naeser, and Palumbo, 1987).
Similarly, the assumption that dementia in patients with ischemic white matter lesions must be related to coexistent AD should be reconsidered in light of data that many such patients have no evidence of cortical amyloid. In one study, for example, 69% of patients with subcortical vascular dementia and severe white matter ischemia had no evidence of cortical amyloid as shown by Pittsburgh compound B (PiB) combined with positron emission tomography (PET) scanning (Lee et al., 2011). Moreover, these individuals performed better on tests of memory than on measures of frontal lobe functions, while those with cortical amyloid on PiB-PET had the opposite pattern (Yoon et al., 2013), again suggesting that white and gray matter lesions exert distinctive effects on cognition. Brain lesions may involve gray matter, white matter, or both, and it would seem that a reasonable and scientific approach is to consider both kinds of lesions in exploring the pathogenesis of neurobehavioral syndromes. While gray matter will not likely be obliged to concede its lofty position as a key neural mediator of human behavior, the white matter is likely to gain respect as a partner in the operations of higher functions, and elucidation of the interplay between the two will greatly expand our understanding.
The timing of neuropathological lesions may offer a helpful perspective as the relative contributions of white and gray matter are considered. As indicated in Chapter 5, the advance of white matter lesions is recognized to be associated with progressive brain compromise in other regions. This principle is most evident in the case of ischemic white matter hyperintensities, which are associated with global brain atrophy over time (Appelman et al., 2009) and produce either ventricular enlargement (Inatomi et al., 2008) or cortical atrophy (Tuladhar et al., 2015). Recent studies have focused on cortical thickness, and longitudinal data are now available to show that the cortex does indeed become thinner over time as white matter hyperintensities accumulate (Smith et al., 2015). The mechanism explaining this progression is likely complex, but the key point may be that early clinical features reflecting white matter disease may blend into those determined by gray matter involvement as time proceeds. This sequence may be typical of many white matter disorders, as will be discussed further later.
Closely related to the issue of coexistent gray matter pathology is the challenge posed by intracortical myelin and its pathology. As reviewed in Chapter 3, small fascicles of myelinated axons coursing through all six layers of the neocortex have been known for more than a century (Nieuwenhuys, 2013). Moreover, some disease states – mainly those involving inflammatory demyelination (Moll et al., 2008) – have been found to feature damage to intracortical myelin. Diseases such as these complicate the distinction between cortical and white matter dysfunction since the white matter of the cortex may be involved along with larger tracts in the subcortical regions. This intracortical myelin damage may secondarily affect the axons, dendrites, and synapses of the cortex so as to produce cortical deficits such as amnesia, aphasia, apraxia, and agnosia. A clinical picture may thus emerge in which neurobehavioral sequelae reflect a commingling of large tract and intracortical white matter involvement.
Currently the importance of intracortical myelin is most evident in MS, where interest in cortical MS has rekindled the supposition that cognitive impairment can be ascribed to gray matter involvement (Lucchinetti et al., 2011). Without doubt, gray matter is affected in MS, and the evidence that this process develops more prominently later in the disease was reviewed in Chapter 6. But even in MS, in which gray matter neuropathology has long been recognized, the WMD model appears justified, at least early in the disease. The resolution of this problem is not yet apparent, but in a thoughtful commentary Feinstein (2007) offered the reasonable statement that whereas white matter disorders may have gray matter involvement that contributes to cognitive impairment and dementia, damage to white matter alone can indeed influence the type of cognitive loss.
Cognitive decline begins early in MS (Amato et al., 2010), and while all patients have white matter lesions, cortical lesions are found with conventional MRI in only 8% of children (Absinta et al., 2011), and, using brain biopsy, only 38% of adults with early disease have cortical lesions (Lucchinetti et al., 2011). Moreover, the cognitive profile of MS resembles other white matter disorders more than AD (Filley et al., 1989), suggesting that tract demyelination is the major determinant of cognitive decline, at least until later in the course. Studies with high field strength MRI have recently shed further light on this issue. Using 7.0 T MRI to examine both gray and white matter in patients with mean illness duration of 9 years, it has been found that the strongest correlates of cognitive dysfunction are white matter lesion volume and lesions that involve the gray matter–white matter junction (“type 1” plaques), suggesting that cognitive decline early in MS primarily results from tract demyelination that involves both large association tracts and the U fibers connecting adjacent gyri (Nielsen et al., 2013). Such studies promise to more firmly establish the relative contributions of white and gray matter neuropathology to the cognitive profile of MS and other white matter disorders.
In this regard, toluene leukoencephalopathy offers a useful perspective. Here the impact of intracortical myelin can be assessed on the basis of how much leukotoxic damage can be observed within the cortex. When the cortex of these patients has been examined, the intracortical myelin has typically been found normal (Rosenberg et al., 1988b; Filley, Halliday, and Kleinschmidt-DeMasters, 2004) and in just one study was intracortical myelin found to be mildly affected (Kornfeld et al., 1994). While more attention to intracortical myelin in toluene leukoencephalopathy is warranted, it would appear at this point that the primary cause for the WMD pattern in this disorder is involvement of large subcortical tracts.
An issue directly pertaining to the concept of WMD is the notion of a threshold effect. How much white matter damage is required to produce dementia? This question is far from straightforward, and indeed the analysis of many other dementia syndromes faces the same general problem. Whether the damage is sustained in white or gray matter, many variables influence the point at which dementia occurs, including the age of the patient; the degree of brain and cognitive reserve before the disorder begins; the presence or absence of other neurologic, psychiatric, or medical problems; and many incompletely understood genetic factors. Nevertheless, investigators have introduced the idea of a threshold effect whereby a certain amount of white matter must be compromised to reach the point where cognitive impairment becomes evident.
The most comprehensive work on this question has come from the study of demyelinative and ischemic white matter lesions as seen on MRI. In MS, it is generally acknowledged that cognitive decline is correlated with increasing lesion load on MRI (Rao, 1995), and an early effort to establish a cognitive threshold proposed that a cerebral plaque burden of 30 cm2 was required to impact cognition (Swirsky-Sacchetti et al., 1992). However, it has also become clear that determining a precise threshold at which dementia begins in MS is complicated by the problem of cortical MS (Lucchinetti et al., 2011), which could hasten the onset of dementia, and cognitive reserve (Sumowski et al., 2010), which could have the opposite effect. In other words, two individuals with MS who have exactly the same white matter lesion burden could have a markedly different risk for dementia.
Work has proceeded in parallel on vascular disease, and an early study of individuals with ischemic white matter hyperintensities concluded that a lesion area of greater than 10 cm2 was associated with disturbances in attentional and frontal lobe skills (Boone et al., 1992). Subsequently, a consensus statement concluded that ischemic involvement of 25% of the cerebral white matter was necessary for the appearance of dementia (Román et al., 1993). Later work with leukoaraiosis (LA) patients also found that 25% could be considered a threshold for the development of cognitive impairment in the domains of executive function, visuospatial ability, and working memory, while declarative memory and language were relatively spared (Price et al., 2005; Libon et al., 2008). Moreover, demented adults with a low burden of LA had impaired episodic memory compared with working memory, whereas those with moderate LA had equal impairment on episodic and working memory, and severe LA was associated with selective impairment of working memory (Price et al., 2005; Libon et al., 2008).
Yet these studies have focused only on macrostructural white matter lesions, and, as will be discussed in the following chapter, microstructural lesions can also be usefully investigated. The question of a threshold effect is clearly influenced by the sensitivity of neuroimaging techniques used to identify white matter pathology, and abnormalities found in the normal-appearing white matter (NAWM) promise to restructure the study of what happens first in white matter disorders, and how these changes may impact cognition. As has been true throughout the MRI era, improvements in technology will permit a more elegant approach to the study of white matter–cognition relationships, and study of the earliest visible manifestations of white matter pathology will be of great interest.
Another issue arises with respect to clinical outcomes of patients with white matter disorders. If it is indeed true that white matter involvement exerts a specific effect on cognition, improvement of white matter lesion burden should produce a salutary clinical effect. One of the ways to assess the strength of the construct of WMD is to consider data on whether the resolution of white matter lesions leads to reversal of the neurobehavioral syndrome produced. That is, if spontaneous recovery from, or treatment of, white matter lesions produces improvement of the clinical deficit, much more credence can be gained as to the neurobehavioral importance of the lesion(s). Spontaneous recovery, however gratifying it may be, is difficult to use for investigating the role of white matter lesions, but outcome studies following treatment are indeed helpful. As will be discussed in Chapter 11, a number of categories of white matter disorder offer examples of specific conditions in which clinical improvement after treatment can be seen in parallel with resolution of or reduction in white matter lesion burden. With further refinement of neuroimaging that can securely identify specific lesions before and after treatment, the impact of these lesions, particularly those that affect one tract and alter one cognitive domain, can be carefully evaluated.
A final issue looming large over all of these unresolved questions is uncertainty revolving around the secure determination of what can be considered normal white matter. As discussed in Chapter 2, the ever-expanding capacity of advanced neuroimaging to reveal subtle aspects of white matter structure calls into question what can be characterized as normal. Whereas the studies reviewed earlier do suggest that white matter disease of any sort can accumulate to produce specific cognitive deficits, it should be recalled that MRI detects only macrostructural white matter lesions, and that the NAWM often harbors microstructural changes that may be of uncertain significance. The work on the NAWM illustrates how the understanding of how white matter contributes to cognitive function is far from complete, and more study using the most sensitive available techniques combined with careful clinical correlation will be needed to clarify these important issues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





