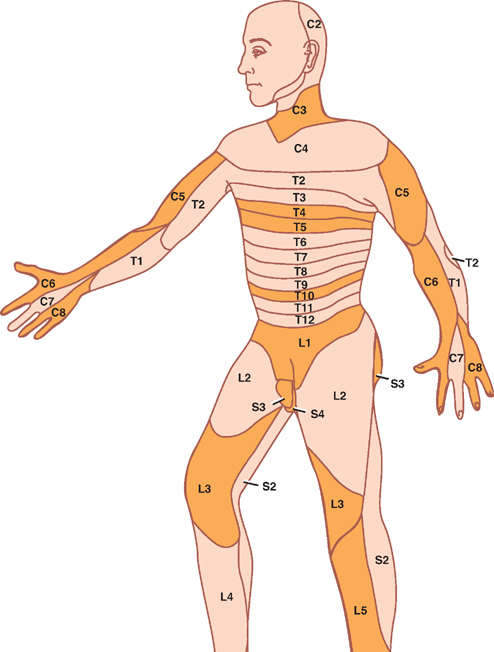
5 Spinal Cord This chapter briefly describes the general gross and microscopic anatomy of the spinal cord. This is followed by a more lengthy discussion of clinical topics, including: The spinal cord forms a nearly cylindrical column that is situated within the spinal canal of the vertebral column. Three meningeal coverings surround the spinal cord: (1) the dura mater, which is attached to the lateral surface of the spinal cord by the denticulate ligament, (2) the arachnoid, and (3) the pia mater (Fig. 5.1). The subarach-noid space contains the cerebrospinal fluid (CSF) and is located between the arachnoid mater and the pia mater. The caudal end of the adult spinal cord is situated at the level of the first or second lumbar vertebra. This is because, following the third month of fetal development, the vertebral column grows faster than the spinal cord (Fig. 5.2). The lumbosacral nerve roots elongate and extend past the caudal end of the spinal cord to form what is known as the cauda equina. The caudal end of the spinal cord tapers off into the cone-shaped conus medullaris, which continues distally as the filum terminale. The filum terminale is a caudal prolongation of the spinal pia mater that courses along with the cauda equina to terminate on the dorsal surface of the coccyx. Fig. 5.1 Spinal cord, nerve roots, and meninges. Fig. 5.2 Differential rate of spinal growth. See Fig. 5.3. Thirty-one pairs of spinal nerves emerge from the human spinal cord: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal. Each spinal nerve is formed in an intervertebral foramen by the union of a ventral root and dorsal root, and each one gives rise to a ventral and dorsal ramus. A ventral root is composed of a series of ventral filaments that carry general somatic efferent (GSE) and general visceral efferent (GVE) fibers. The cell bodies of origin of these axons constitute the motor nuclei of the ventral horn of the spinal cord. More recently it has been shown that the ventral roots contain afferent (sensory) fibers as well. The dorsal roots comprise a series of dorsal filaments that carry general somatic aE fferents (GSA) and general visceral afferents (GVA) that originate in the dorsal root ganglion (spinal ganglion). Distally, the ventral and dorsal roots converge to form a spinal nerve, which contains all four functional components (GSA, GVA, GSE, and GVE). As the spinal nerve emerges distally from the intervertebral foramen, it divides into a dorsal and ventral ramus. The dorsal ramus branches into peripheral nerves that innervate the paraspinal muscles and the skin of the back; the ventral ramus divides into peripheral nerves and plexuses that innervate the muscles and the skin of the ante-rolateral body wall, the limbs, and the perineum. Finally, the gray and white rami connect the spinal nerves to the sympathetic trunk. Fig. 5.3 Spinal nerve. See Fig. 5.4. A dermatome comprises that area of skin that is innervated by the sensory fibers of an individual spinal nerve. However, the distribution of a single dermatome overlaps adjacent dermatomes, and thus destruction of a single spinal nerve does not produce clinically evident anesthesia. A dermatomal map is shown in Fig. 5.4. Key dermatomes include the following: C3: Neck C5: Deltoid region C6: Radial forearm and thumb C8: Ulnar aspect of hand and little finger T4-T5: Nipple T10: Umbilicus L1: Groin L3: Knee L5: Dorsal surface of foot and great toe S1: Lateral surface of foot and little toe S3-S5: Genitoanal area Although their pattern of innervation is less obvious than in the case of sensory fibers, the motor fibers of a spinal nerve generally innervate those muscles that lie beneath the corresponding dermatome. Each spinal nerve supplies several voluntary muscles; likewise, each muscle is innervated by several spinal nerves. Some of the more clinically relevant muscles and their innervation include the following: Biceps brachii: C5-C6 Triceps: C6-C8 Brachioradialis: C5-C7 Intrinsic muscles of the hand: C8-T1 Thoracic musculature: T1-T8 Abdominal musculature: T6-T12 Quadriceps femoris: L2-L4 Gastrocnemius: S1-S2 Muscles of the perineum, bladder, and genitals: S3-S5 Fig. 5.4 Segmental innervation of the body. The internal structure of the spinal cord is organized into two discrete areas: (1) an inner core of gray matter containing cell bodies and mostly unmyelinated fibers and (2) an outer coat of white matter carrying both myeli-nated and unmyelinated fibers. The distribution of gray and white matter in the spinal cord varies with the level of the spinal cord. For example, at cervical and lumbar levels that correspond to the innervation of the limbs, there is an increased proportion of gray matter. Furthermore, there is a decreased amount of white matter present at lower levels of the cord because the descending fibers exit the cord caudally, whereas ascending fibers enter rostrally. See Fig. 5.5. The centrally located H-shaped gray matter is composed of nerve cells and their processes, neuroglia, and blood vessels. A small central canal, obliterated in places, is lined by ependymal epithelium. Each side of the gray matter is composed of (1) a dorsal horn, extending pos-terolaterally, (2) a ventral horn, extending anteriorly, and (3) an intermediate gray, which connects the dorsal and ventral horns. A small lateral horn is formed in the intermediate gray of the thoracic and upper lumbar segments. The dorsal horn is primarily composed of the following four cell groups: Two cell groups contribute to the intermediate gray: The principal cells of the ventral horn are motor neurons. The motor activity of these neurons is subject to the influences of (1) interneurons, (2) dorsal root afferents (which act in spinal reflexes), and (3) the descending tracts of the brain. Significantly, the neurons situated in the ventral horn are somatotopically organized in two ways. First, the neurons of the ventral horn that innervate the flexor muscles lie dorsal to those that innervate the extensor muscles; second, the neurons that innervate the muscles of the hand lie lateral to those that innervate the trunk. The logic of these relationships is made clear when one considers that the descending pathways associated mainly with the control of the flexor musculature, such as the corticospinal and rubrospinal tracts, are located dorsally in the lateral funiculus. On the other hand, the descending pathways associated mainly with the anti-gravity muscles (i.e., generally the extensor musculature) are situated in a more ventral position. Two types of motor neurons are distinguished on the basis of the diameter of their axons. Large a motor neurons supply ordinary (extrafusal) muscle fibers of skeletal muscles; smaller 7 motor neurons innervate intrafusal fibers of the neuromuscular spindles. As mentioned, the ventral horn contains the following two groups of neurons: As an alternative classification, the gray matter of the spinal cord has also been divided into 10 layers of neurons, known as Rexed’s laminae. Among the most important groups of laminae are the following (Fig. 5.6): Fig. 5.5 Components of the gray matter of the spinal cord. Fig. 5.6 Organization of the gray matter of the spinal cord. See Fig. 5.7. The white matter of the spinal cord may be divided into ventral, lateral, and dorsal funiculi. These funiculi contain the ascending and descending fiber bundles (fasciculi or tracts) that transmit signals both within the spinal cord and between the spinal cord and the brain. Fig. 5.7 illustrates the relative position of the major tracts. Fig. 5.7 White matter of the spinal cord. See Fig. 5.8. Two dorsal or posterior white columns, the fasciculus gracilis and the fasciculus cuneatus, occupy the dorsal fu-niculi. The fasciculus cuneatus begins at T6, below which there is only the fasciculus gracilis. Separated from each other by a septum, these tracts mediate position sense, vibratory sense, and discriminative touch. The tracts are organized somatotopically. Those fibers that transmit sensation from the legs are located medially (fasciculus gracilis). Those fibers that transmit sensation from the arms are located laterally (fasciculus cuneatus). The anterior spinothalamic tract lies anteromedial in relation to the lateral spinothalamic tract. It is concerned with light touch and pressure sensation. Fig. 5.8 Somatotopy of spinal tracts. See Fig. 5.9. Fig. 5.9 Corticospinal tracts. See Fig. 5.10. The blood supply of the spinal cord is primarily derived from one anterior and two posterior spinal arteries. Both of these arteries are branches of the distal vertebral artery. The anterior spinal artery arises from an anastomosis of two branches from the vertebral artery. The anterior spinal artery extends from the medulla to the tip of the conus medullaris. It supplies the anterior two thirds of the spinal cord. The posterior spinal arteries arise as paired branches from the intracranial vertebral artery, although they may also arise from the posteroinferior cerebellar arteries. They extend the length of the spinal cord and supply its dorsal third. At the conus medullaris, the anterior and posterior spinal arteries anastomose. Throughout their course, both arteries receive supply from the radiculomedullary arteries, most of which are branches of the descending aorta. Three regions in the vertical axis of the spinal cord are distinguished by the relative richness of their arterial supply and are related to the distribution of the anterior spinal artery. Fig. 5.10 Blood supply of the spinal cord. See Fig. 5.11. Thrombotic occlusion of the anterior spinal artery is the most common vascular syndrome of the spinal cord. Most frequently it occurs in watershed or boundary zones, such as the midthoracic region (T3–T8). It has four essential features: This syndrome is rare. Manifestations may include loss of position, vibratory, and light touch sensation below the level of the lesion (due to involvement of the dorsal columns) with preservation of motor, pain, and temperature modalities. Fig. 5.11 Anterior spinal artery syndrome. See Fig. 5.12. Spinal tumors fall into one of three categories: (1) ex-tradural extramedullary, (2) intradural extramedullary, and (3) intradural intramedullary. Clinically, extradural and intradural extramedullary tumors are difficult to differentiate. The following discussion therefore describes both extradural and intradural extramedullary tumors together. Extramedullary tumors tend to produce pain in a radicular distribution. Frequently, this pain is associated with tenderness to palpation in the vertebral column. Ironically, this pain and tenderness are often accompanied by loss of normal pain and temperature sensation. The parapare-sis associated with an extramedullary lesion tends to be spastic, and this spasticity persists even after paraplegia develops. There is little or no muscle atrophy associated with an extramedullary lesion. Muscle fasciculations are common. Trophic disturbances of the skin are usually absent. Bowel and bladder disturbances tend to occur late, unless the tumor is located in the sacral region. Radicular pain in intramedullary tumors is rare. Dys-esthesias and paresthesias are common, as is dissociated sensory loss and sacral sparing (due to the somatotopic organization of the spinothalamic tract). Paraparesis is spastic in only 50% of cases and when present is less pronounced than in extramedullary tumors. Muscle atrophy is common, and muscle fasciculations are rare. Trophic disturbances of the skin are seen fairly frequently. Bowel and bladder disturbances tend to occur early. Fig. 5.12 Spinal tumors. Lesions at the level of C3–C5 may involve the phrenic nucleus, which innervates the diaphragm. Diaphragmatic paralysis causes limited lateral expansion of the lower rib cage during respiration, resulting in respiratory compromise. Upper cervical spinal cord lesions may be associated with bradycardia (due to interruption of fibers ascending to the cardiovascular center of the medulla). Such lesions may also be associated with hypotension (due to interruption of descending sympathetic fibers). The simultaneous presence of hypotension and bradycardia serves to distinguish hypotension due to loss of sympathetic tone from hypovolemic hypotension because the latter is typically associated with tachycardia. The former is seen during spinal shock following spinal cord injury. See Fig. 5.13. Sympathetic fibers originate in the hypothalamus and descend through the brainstem to reach the interme-diolateral gray matter of the C8–T2 spinal segments of the spinal cord (first-order neuron). Subsequent fibers are projected to the superior cervical ganglion (second-order neuron) and finally to the superior tarsal muscle, the dilator pupillae muscle, and the sweat glands of the face (third-order neuron). Injury to the C8–T2 spinal segments may result in Horner syndrome, which consists of (1) ptosis (due to denervation of the superior tarsal muscle), (2) miosis (due to denervation of the dilator pupillae muscle), and (3) anhidrosis (due to denervation of the sweat glands of the face).
Gross Anatomy
General Features


Spinal Nerves

Segmental Innervation of the Body
Skin
Muscle
Internal Structure
Gray Matter
Dorsal Horn
Intermediate Gray
Ventral Horn


White Matter
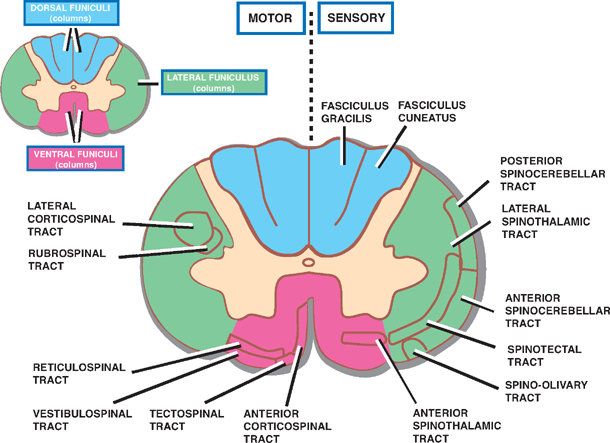
Ascending Tracts
Dorsal Funiculi
Ventral Funiculi
Lateral Funiculi

Descending Tracts
Ventral Funiculi
Lateral Funiculi

Blood Supply of the Spinal Cord

Vascular Syndromes of the Spinal Cord
Anterior Spinal Artery Syndrome
Posterior Spinal Artery Syndrome

Spinal Tumors
Extramedullary Tumors
Intramedullary Tumors

Autonomic Disturbances in Spinal Cord Lesions
Respiratory Disturbances
Cardiovascular Disturbances
Horner Syndrome

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








