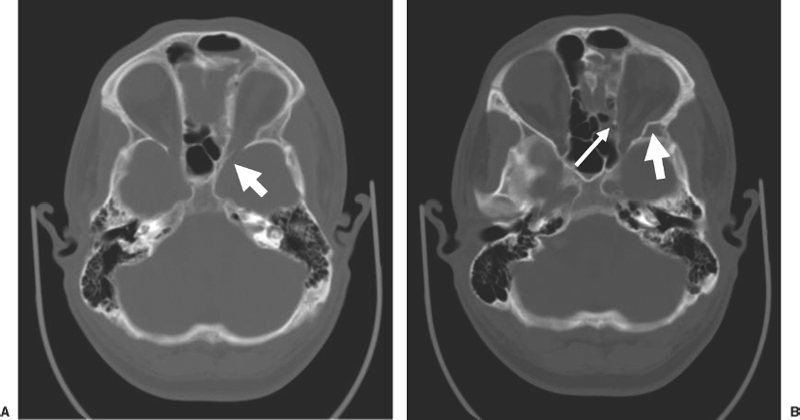
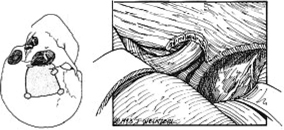
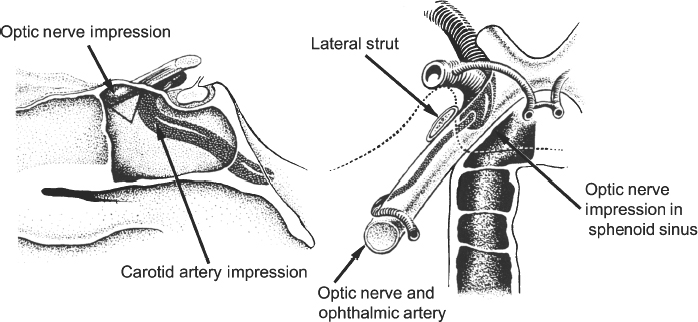
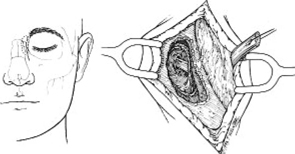
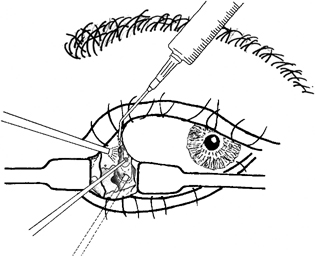
13 Acute Bony Decompression of the Optic and Facial Nerves Jason Heth, Christine Nelson, and H. Alexander Arts Neurosurgical consideration of cranial nerve decompression following head and facial trauma arises primarily in the setting of optic (II) and facial (VII) nerve injury. The course of these cranial nerves through bony foramina in the frontal and temporal regions makes them particularly susceptible to compromise following fracture or deformation of the basal cranial vault. The role of surgical decompression in these injuries has been widely debated. The use of high-dose steroids has improved outcome without the need for surgical intervention in some cases. Management has been dictated by interpretation of retrospective data. Despite the increased push in medicine toward randomized trials and outcomes studies, prospective data regarding optimal treatment of traumatic optic and facial neuropathies have not been obtainable. Each surgeon’s treatment of such neuropathies requires his or her best judgment in an analysis of the available retrospective data. Disturbances of the visual system have been described in 2% to 11% of patients with head injury1–6 and in up to 67% of patients with facial fracture.7 Optic nerve injuries not associated with penetrating trauma or rupture of the globe are less frequent. Indirect optic nerve injuries are estimated to occur in 0.5% to 1.5% of closed cranial trauma8,9 and in up to 3% of patients with facial fractures.7 Patients with orbital trauma may present with decreased or absent visual acuity, afferent pupillary defects, blindness and concomitant ophthalmoplegia (orbital apex syndrome), proptosis, mydriasis, and ptosis (superior orbital fissure syndrome).10–12 The optic nerve is most susceptible to injury within rigid confines of the optic canal, where it is fixed to the meninges and periosteum.13,14 Patients with immediate blindness following trauma are generally believed to have suffered avulsion of the optic nerve, and have a dismal prognosis for return of visual acuity. Partial preservation of vision suggests compromise of the optic nerve without avulsion and a reasonable chance of maintaining vision in the injured eye. High-dose steroids are believed to improve the outcome of visual acuity in such patients.15–17 The role of surgical intervention for decompression of the optic nerve within its canal has been controversial. The clinical outcome following posttraumatic decompression of the optic nerve varies significantly.18–21 The wide spectrum of injuries and pathophysiologic mechanisms that may produce optic nerve dysfunction further complicates the interpretation of these results. High-dose steroids are frequently administered as a first line treatment.22–24 Most authors agree that a documented decline in visual acuity following serious head injury or facial fracture warrants consideration of optic nerve decompression, particularly if there has been an initial improvement on high-dose steroids followed by further decline.17,20,25 If surgery is undertaken, the approach is determined by the pathology of the lesion and associated injuries. Fronto orbital,17,20,25 lateral orbital,26–29 and transethmoidal approaches5,15,18,30–33 have all been described. There continue to be no large prospective clinical studies comparing surgical to nonsurgical management, and the surgeon confronted with a patient with declining visual acuity following head or facial injury is therefore best guided by a critical review of the available retrospective studies. The orbit is an irregular pyramid with the apex pointed posteriorly.34 The roof has a concave shape formed by the orbital portion of the frontal bone, which articulates posteriorly with the lesser wing of the sphenoid and laterally with the zygomatic bone. The lateral wall is formed by the orbital part of the frontal bone, the greater wing of the sphenoid, and the zygomatic bone. Posteriorly, the lesser wing of the sphenoid is separated from the greater wing by the superior orbital fissure, which opens into the middle cranial fossa. The frontal processes of the zygomatic bone and the zygomatic process of the frontal bone form the lateral margin of the orbit. The medial margin of the orbit is formed by the frontal process of the maxilla and the maxillary process of the frontal bone meeting at the frontal maxillary suture. The medial wall is formed anteriorly by the lacrimal bone and more posteriorly by the ethmoid and adjacent frontal and maxillary bones. Posteriorly, the ethmoid meets the sphenoid where the two roots of the lesser wing enclose the optic canal. The floor of the orbit is formed by the orbital surface of the maxilla, the zygomatic bone anteriorly and laterally, and the orbital process of the palatine bone near the orbital apex. This forms the roof of the maxillary sinus.The anterior part of the zygomatic bone and maxilla are thickened and form the inferior margin of the orbit. The optic canal is an elliptical structure that is wider horizontally at its cranial opening and wider vertically at its orbital opening.14 The canal enters the orbit through the most posterior part of the medial wall, and transmits the optic nerve and ophthalmic artery. The canal traverses the sphenoid bone into the orbit at a 39.1-degree angle.35 The canal has the largest cross-sectional area at its cranial opening and the smallest area in its middle.36 The superior orbital fissure, between the lesser and greater wings of the sphenoid, opens into the back of the orbit and separates the posterior part of the roof and lateral wall. This fissure curves downward and medially to widen near the apex of the orbit. It transmits the superior and inferior ophthalmic veins, the frontal and nasociliary nerves, as well as the superior and inferior divisions of the oculomotor (III) nerve, the trochlear (IV) nerve, and the abducens (VI) nerve. The superior orbital fissure lies lateral and slightly inferior to the optic canal. The primary optic vesicles (vesicular invaginations of the cephalic end of the primitive forebrain) ultimately form the optic nerves, retina, posterior epithelium of the ciliary body, and iris.34,37 Axons of retinal ganglion cells converge on the optic disk 3 mm medial to the sagittal meridian and slightly above the horizontal meridian. These fibers become myelinated at the optic disk, accounting for the white strands occasionally seen on the nerve head with funduscopic examination. Optic disk fibers gather into bundles, which then traverse the sclera, forming the meshlike lamina cribrosa. These fibers coalesce into the compact optic nerve and are encased in the three layers of the meninges, passing posteriorly and slightly medially to enter the optic canal within a tendinous ring (annulus of Zinn) that serves as a point of attachment for the extraocular muscles. Approximately 1 cm posterior to the globe, the central retinal artery and vein of the retina pierce the inferior surface of the optic nerve and coverings to supply the retina. Within the optic canal, the ophthalmic artery lies lateral to the sphenoid sinus and gives rise to the central retinal artery. The optic nerve can be anatomically divided into four parts: intraocular, orbital, intracanalicular, and intracranial.20,37 The intraocular portion is ~1 mm long and 1.5 mm in diameter, consisting primarily of unmyelinated fibers passing from the optic disk to the lamina cribrosa. The orbital portion is 3 to 4 mm in diameter and 20 to 30 mm long. It pursues a slightly sinuous course to the optic canal to allow for movement of the globe in the orbit. The orbital optic nerve is invested by a sheath of dura, arachnoid, and pia, which becomes continuous with the sclera at the lamina cribrosa. The dura extends back to the cranial cavity, and the pia extends to the optic chiasm. The subarachnoid and subdural spaces are continuous with the intracranial cavity. Posteriorly, the optic nerve sleeve is crossed obliquely by the nasociliary nerve, the ophthalmic artery, the superior ophthalmic vein, and the superior division of the oculomotor nerve. The intracanalicular segment of the optic nerve is 4 mm in diameter and ~10 mm in length. At the entrance of the optic canal, it is encased by a tendinous ring (annulus of Zinn), which is the origin of insertion of the extraocular muscles. Within the optic canal, the optic nerve is accompanied by the ophthalmic artery, which lies inferiorly. Medially, a thin shelf of bone separates the canal from the sphenoid sinus. Superior to the nerve, the three sheaths of meninges are fused, affixing the nerve to the periosteum above. This tethers the optic nerve within the optic canal, thereby preventing movement of the intracanalicular nerve. The intracanalicular subarachnoid and potential subdural spaces are present only inferior to the nerve. The blood supply for the intracanalicular optic nerve arises from the ophthalmic artery. The artery gives off several small branches which first run in the dura, enter the pia, and supply the optic nerve by a capillary network surrounding the optic nerve.36 The intracranial portion of the optic nerve is 4 to 6 mm in diameter and ~10 mm long. It lies just medial to the internal carotid, and superior to its branch point for the ophthalmic artery. Just prior to the optic chiasm, the anterior cerebral artery crosses the optic nerve superiorly. Optic nerve compression may be associated with a variety of pathologic conditions. Most commonly, compression is associated with fractures of the orbital complex, including the ethmoid bones, optic canal, frontal orbital plate, orbital floor, sphenoid bone, lateral orbital wall, and “blow-in” fractures of the orbit.4,5,10,17,26,38–40 In some cases of posttraumatic optic nerve dysfunction, no fractures can be identified.15,20 Many authors believe that compressive edema or vascular insufficiency is the most likely pathologic mechanism of posttraumatic optic neuropathy.41,42 Hemorrhage25,43,44 into the optic nerve sheath has been described, as well as laceration of the intracanalicular optic nerve by fracture fragments.18,19,37 Walsh25 classified optic nerve lesions occurring in conjunction with head injuries into primary and secondary insults. A primary insult refers to alterations that occur with impact forces such as hemorrhage, shearing of nerve fibers, and contusion. Secondary insults represent the delayed effects of impact, such as edema, necrosis secondary to local vascular compromise, or infarction secondary to thrombosis of the ophthalmic artery. Steinsapir and Goldberg reviewed the molecular events initiated in secondary injury of the optic nerve in detail.45 Kline et al20 described six categories of indirect optic nerve injury: laceration, bone deformation of fracture, vascular insufficiency, concussion, contusion, and hemorrhage. Lacerations or stretch avulsion injuries are usually seen in the area of the cranial opening of the optic canal, and are most likely secondary to a tethering effect of the fixed intracanalicular portion and the relatively mobile brain and globe. Direct compression of the optic nerve by bony fragments, with subsequent visual improvement following decompression, has often been reported. The mechanism of deformation without fracture was addressed experimentally by Anderson et al,15 using holographic interferometry to evaluate stress in the optic canal with application of external forces. Their work suggested that significant forces were concentrated adjacent to the optic canal with frontal injuries, and that deformation of the canal could result without gross fracture, causing contusion of the optic nerve. Pathologic evidence of vascular insufficiency with resultant optic nerve infarction was reported by Hughes41 and Ramsay,42 supporting the explanation that the mechanism involved in indirect optic nerve injury is vascular compromise. Their findings were localized to the intra-canalicular portion of the optic nerve, supporting the idea that this is the region susceptible to compressive ischemia following injury. Hemorrhage within the optic sheath or optic nerve has been detected intraoperatively and in postmortem studies by Pringle,46 Walsh,25 and Niho et al.44 In the operative series reported by Hammer and Ambos,43 four patients were noted with optic nerve sheath hematoma, all of which improved postoperatively, following evacuation of the blood clot. In summary, a variety of pathologic mechanisms have been proposed, each supported by intraoperative or postmortem findings in selected patients. Most likely, a variety of mechanisms are responsible for posttraumatic optic neuropathy, which complicates efforts to compare therapies. The effect of surgical intervention on the prognosis of each type of injury is unknown. Anecdotal evidence supports decompression in cases of known intradural hemorrhage, bony compression, and possibly compressive ischemia secondary to edema in the intracanalicular portion of the optic nerve. Concomitant head and facial injuries often complicate the initial evaluation of visual acuity following trauma. Indirect injuries to the optic nerve can be classified into anterior and posterior types.20 Visual acuity may be unavailable, absent, diminished, or preserved in either type of injury. Anterior injuries involving the intraocular portion of the optic nerve generally present with funduscopic abnormalities, including diffusely swollen optic disks, retinal edema secondary to central retinal artery disruption, or total avulsion of the optic nerve head. Posterior injuries are defined as optic nerve dysfunction in the absence of funduscopic abnormalities, and are generally the result of insult to the optic nerve in the optic canal. Degenerative funduscopic abnormalities such as optic disk pallor or loss of the retinal nerve fiber layer are not apparent on initial evaluation but may be observed several weeks after injury. Although described separately, these two types of injury may occur in combination. An initial assessment of visual acuity of each eye separately is critical, if possible. Ideally, this is accomplished using a formal acuity chart. The ability to read printed material, count fingers, or simply perceive light should be documented in each eye. The ability to count fingers and perceive light can be further described in finer increments (for example, counts fingers at 2 ft or perceives a bright light at 2 ins from the cornea). In patients with a diminished level of consciousness, absence of an afferent pupillary defect and an aversive response to bright light suggest intact light perception. Documented progressive deterioration in visual acuity following head trauma should prompt consideration of optic nerve decompression. Decreased direct pupillary response to light is cited as the most reliable index of optic nerve compromise, according to Edmund and Godtfredson.39 With unilateral optic nerve injury, the initial pupillary size is equal bilaterally; however, upon direct light stimulation of the impaired eye, pupillary constriction occurs more slowly and to a lesser degree-or not at all-than if the stimulus had been applied to the normal eye. This difference in pupillary constriction, constituting a relative afferent pupillary defect (RAPD), carries the eponym the Marcus Gunn pupil.47 It is important to note, however, that if bilateral optic nerve injury is present, an RAPD may be more subtle or not be present. Assessment of the visual pathways is often difficult when the level of consciousness is decreased in patients with severe head injuries, as previously noted. Visual evoked responses and electroretinograms may provide additional information to guide long-term clinical management. From a practical standpoint, limited availability and technical difficulty associated with testing in the setting of acute trauma may limit the usefulness of monitoring visual evoked response and electroretinography. Good correlation between the initial visual evoked response and ultimate visual acuity has been described.48–50 Furthermore, in some settings, the visual evoked response may be superior to clinical assessment in determining outcome of visual acuity. Greenberg et al51 described a 90% predictive accuracy with visual evoked response testing, as opposed to 30% with clinical examination, in a series of patients with retrobulbar dysfunction examined within 3 days of insult, and again at 3 months or longer. The association of optic nerve injury with facial and orbital fractures and the reported cases of improvement following removal of compressive bony fragments mandates thorough neuroimaging investigation in cases of compromised visual acuity. The procedure of choice is thin-section computed tomography (CT), as it allows resolution of the bony detail of the orbital apex region (Fig. 13-1).13,52,53 Axial and coronal CT scans can be obtained, and these may demonstrate orbital fractures and bony fragments. Reconstructed images including three-dimensional views can also assist in detailed evaluation of facial and orbital injuries. It must be remembered, however, that it has been reported that compressive bony fragments not observed on preoperative CT imaging have been found at operation.54 Magnetic resonance (MR) imaging may demonstrate soft-tissue injury and hemorrhage or hematoma within the dural sheath or optic nerve. Figure 13-1 Axial CT of a 24-year-old man who sustained orbital apex and optic foramen fractures. (A) Orbital apex fractures in region of optic foramen and superior orbital fissure (white arrow). (B) Fractures in medial (thin arrow) and lateral (large arrow) orbital wall fractures. The lateral orbital wall fracture is impinging the lateral rectus muscle. Corticosteroids have assumed a prominent role in the management of traumatic optic nerve injury. This occurred at the same time trials using corticosteroids in the management of spinal cord injury were suggesting a beneficial treatment effect. Although a range of dosing has been studied, the most common regimen calls for an initial loading dose of 30 mg/kg, followed by 15 mg/kg infused every 6 hours for 3 days. Several series utilizing corticosteroids reported substantial improvement of visual acuity.15–17,23,24,55–57 In one meta-analysis, any treatment (corticosteroids, extracranial decompression, or corticosteroid and extracranial decompression) improved visual acuity more than observation alone.55 No difference between treatment modalities could be found. In contrast, there are other series which do not show any benefit for steroids over observation, most prominently the report from the International Optic Nerve Trauma Study.58 The International Optic Nerve Trauma Study was conceived to compare extracranial optic nerve decompressive surgery with corticosteroids to corticosteroids alone. Unfortunately, enrollment of eligible patients was insufficient to provide statistical validity. The study was changed to an observational study. The results of this study suggest no differences in traumatic optic nerve injury outcome between observation, corticosteroid, and decompressive surgery groups. Another consequence of this study is that there remains no randomized prospective study to determine the optimal treatment for traumatic optic nerve injury. A similar trial will likely not occur any time soon. An afferent pupillary defect with a normal funduscopic examination suggests injury to the optic nerve. Once the diagnosis of optic nerve compromise is made, decisions regarding appropriate management must be addressed. The time since injury and the degree of progression of visual deficit should be noted. Complete loss of vision immediately following injury generally carries a dismal prognosis and, although cases of recovery have been described,18,19 most authors advocate treatment with corticosteroids in these circumstances.17,20,27 Several related but subtly different indications have been utilized for individuals with some degree of preserved vision. One of the more common indications recommends optic nerve decompression if visual function deteriorates during or after corticosteroid treatment. Others extend this indication by offering decompression if visual function does not improve during corticosteroid treatment. Still others advocate decompression if any optic nerve hematoma or orbital fracture causing an optic canal compressive fragment is discovered on imaging. Debate continues regarding the optimal time for decompression. Several reports document improved visual outcome for decompression performed within 7 days of injury compared with decompression performed after 7 days.22,24,56 Others have not found this time period to be a significant factor in visual outcome.54,59,60 Thakar and colleagues61 reported improvements in visual function for patients undergoing decompression up to 1 year after injury and recommend decompression for patients with traumatic optic neuropathy with decreased vision persisting up to 1 year after injury. Another factor that may be important is the presence of orbital, orbital apex, or optic canal fractures. Again there is no agreement as to whether the presence of such fractures is prognostic of visual outcome. Some reports document worse visual outcomes in patients suffering orbital apex fractures,22,24,60 while others find no difference in outcomes between patients with and without such fractures.23,54–56,58 Some authors who found no difference admit that small sample sizes may have limited their power to detect a difference between the two groups. When surgical intervention is considered, the approach is dictated by the mechanism and location of the insult, as well as associated injuries. Successful optic nerve decompression requires removal of one half of the circumference of the bone along the entire length of the optic canal. If a fracture is present, the approach is best selected based on the type of fracture and the direction of compression, if this can be determined by preoperative radiologic studies. In the absence of clear pathology, adequate decompression of the canal should be possible regardless of the direction of approach. The intracranial frontal orbital approach to the optic canal, as described in 1922 by Dandy62 for orbital tumors, is frequently utilized. This exposure is particularly useful when associated intracranial pathology requires surgical attention. The transethmoid approach, originally described in 1926 by Sewall63 and popularized and modified by Niho et al,44 Fukado,19 Sofferman,64 and others,5 avoids a formal craniotomy and provides exposure of the medial orbital apex with minimal morbidity. When the compression is secondary to fractures of the lateral orbital wall, the lateral facial or lateral temporal approach has been successfully used to decompress the optic nerve and canal.26–29 This approach provides wide access to the lateral orbit, including the region of the superior orbital fissure, with minimal retraction of the orbital contents. Variations on these techniques have been described for use in selected cases. The transfrontal approach to visualize the optic nerve and chiasm is very familiar to neurosurgeons (Fig. 13-2). Access to the optic chiasm, intracranial optic nerve, and posterior aspect of the optic canal allows direct inspection of these structures in cases of frontotemporal injury.2,38,41,62 Dural tears and orbital plate fractures can be repaired, and associated intracranial pathology can be addressed concurrently. The optic canal can be unroofed and the dural sheath of the optic nerve incised to allow adequate decompression and inspection of the optic nerve. This technique has been described and utilized by many authors and has been labeled by Sofferman as “the standard surgical technique upon which virtually all reported series of optic nerve decompression are based.”64 Figure 13-2 Transfrontal optic nerve decompression via a right frontal craniotomy. The approach to the optic canal is shown. The frontal lobe has been retracted and a portion of the bony optic canal unroofed, allowing inspection of the optic nerve and dural sheath. Figure 13-3 The lateral wall of the sphenoid sinus. The optic nerve and carotid artery create impressions on the lateral wall. The carotid artery is posterior and inferior in relation to the optic nerve, therefore optic nerve exposure should begin anterior and superior in relation to the optic nerve impression. (Figure adapted from Goldberg RA, Steinsapir KD. Extracranial optic canal decompression: indications and technique. Ophthal Plast Reconstr Surg 1996; 12:163–70.) Transethmoidal approaches to the optic canal have assumed a prominent role in optic nerve decompression for trauma. There are several reasons for this. Transethmoidal approaches obviate the need for craniotomy, which some surgeons feel carries a higher rate of risks. Transethmoidal approaches require less time in fashioning the approach and take less time to perform. Finally, these approaches tend to be less invasive, particularly when performed endoscopically, and coincide with trends toward minimally invasive surgery. There are two particular drawbacks to these approaches, however. Foremost, the carotid artery travels near the optic nerve adjacent to the lateral wall of the sphenoid sinus (Fig. 13-3). Removal of bone over the optic nerve must be meticulous to avoid injury to the carotid artery. Carotid artery laceration in this location may be a fatal event because there is no vascular control. Another risk in these approaches is cerebrospinal fluid (CSF) leak. This occurs through two primary events. First, any exposure or drilling that is too superior risks traversing the planum sphenoidale dura and can result in CSF rhinorrhea. Second, the optic nerve sheath may either be lacerated from the traumatic event or incised as part of the procedure. Such openings in the optic nerve sheath can also lead to CSF leak. Transethmoidal approaches may be subdivided into transfacial, endoscopic transnasal, and transconjunctival. The transfacial transethmoidal approach to the medial aspect of the orbital apex includes a facial incision. A vertical incision is made just medial to the medial canthus of the eye (Lynch incision), dividing the medial palpebral ligament (Fig. 13-4). An oval portion of bone (1 x 1.5 cm) near the junction of the maxillary, ethmoidal, and frontal bones is resected, exposing the ethmoidal sinus. After removal of the mucous membranes and bony septa of the sinus, the prominence of the optic canal is found deep in the lateral recess of the sinus. If the thin medial wall of the sinus has been fractured, the bony fragments are carefully removed. The optic canal is decompressed along its medial wall. There are no consistent recommendations regarding incision of the optic nerve sheath, some authors incising the sheath,33,54,64,65 and some avoiding incision,23,66 and others undecided.24,30,67 This approach is hampered by limited visibility and a narrow angle of approach to the optic canal. A modified sphenoethmoid approach has been extensively described by Sofferman64 to improve the angle of approach. Figure 13-4 A left transethmoid optic nerve decompression. (A) The incision and area of bone resection are outlined. (B) The contents of the ethmoid sinus are removed, and the optic canal is found passing obliquely in the lateral recess of the ethmoid sinus. A portion of the bony optic canal has been unroofed to allow inspection of the optic nerve and sheath. The divided medial palpebral ligament is retracted with a suture. Endoscopic transnasal transethmoidal approaches begin with an endoscopic ethmoidectomy. Once the sphenoid sinus is identified, it is entered. The lateral wall of the sinus is examined to find the prominences overlying the optic nerve and carotid artery (Fig. 13-3). Bone removal begins over the thin lamina papyracea and proceeds posteriorly. Drilling should occur under continuous irrigation to prevent thermal injury to the optic nerve. The bone should be drilled to a thin remnant, which is then carefully removed to prevent carotid artery injury. The optic nerve sheath may be incised, as previously discussed. Any evidence of CSF leak should be treated with any of many methods available (fibrin glue, dural substitutes, cadaveric fascia lata, etc.) Endoscopic approaches have also utilized transconjunctival exposure to increase the orbital exposure. Approaches through the orbit alone24 (Fig. 13-5) and through the orbit and nasal cavity together32 have both been described as increasing visualization.
Traumatic Injury to the Optic Nerve: Overview
Anatomy of the Orbit
Anatomy of the Optic Nerve
Pathophysiology of Optic Nerve Injury
Evaluation of Traumatic Optic Nerve Injury
Neuroimaging Assessment
Management of Traumatic Optic Nerve Injury
Nonoperative Treatment and Comparisons with Operative Treatment
Indications for Optic Nerve Decompression
Choice of Surgical Approach
Transfrontal Approach
Transethmoidal Approach
Acute Bony Decompression of the Optic and Facial Nerves
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








