11 Cerebral Infectious Processes Walter A. Hall The development of an intracranial infection is dependent on the virulence of the offending organism and the immune system of the host.1 Other factors which will influence whether a central nervous system (CNS) infection becomes clinically relevant include the size of the infectious inoculum, whether there is an underlying disease suppressing the immune system, and whether or not immunosuppressive medications are being administered. An emerging problem that can contribute to neurosurgical morbidity and mortality is the development of resistance to treatment by the organism. Despite the availability of new, potent therapeutic agents for the treatment of CNS infection, meningitis, epidural abscess, subdural empyema, and brain abscess still represent serious neurosurgical problems requiring immediate attention. Solid organ transplantation and the increasing length of survival for some cancer patients have influenced the growing number of infections involving the CNS. Computed tomography (CT) and magnetic resonance (MR) imaging now allow clinicians more readily to recognize the presence of a CNS infection. Stereotactic techniques allow neurosurgeons to sample deep, remote areas of the brain that may harbor infection. Improved clinical outcomes are associated with rapid medical and surgical intervention after the isolation of the causative organism and decompression of neural tissue. The infectious processes encountered by neurosurgeons will be the focus of this chapter, with emphasis on medical and surgical management. An intact blood-brain barrier (BBB) is essential to prevent CNS infection. The way organisms cross the intact BBB to invade the CNS remains unknown, but the choroid plexus is the first site of inflammation. In trauma, the antimicrobial inoculum introduced into the CNS must overwhelm the immune system for an infection to develop. For a postoperative wound infection to occur, -100 000 bacterial organisms per gram of tissue are necessary.2 Once organisms have entered the CNS, meningitis can develop due to low levels of complement and immunoglobulins.1–4 Reasons for the low levels of complement found in the cerebrospinal fluid (CSF) during infection are the inability to cross the BBB, enhanced clearance, low production rates, varying degrees of inflammation, and degradation.1,2 The most important bacterial virulence factor that leads to meningitis by inhibiting phagocytosis and resisting complement activity, thereby allowing for organism replication and survival in the bloodstream, is encapsulation.3 The alternative complement pathway and the terminal complement components C5 to C9 represent the immune response to the bacterial capsule.4 Inflammation in the subarachnoid space enhances passage across the BBB of white blood cells (WBC) and antibiotics, such as the penicillins. The location for BBB permeability by neutrophils is at the level of both the choroid plexus epithelium and the microvascular endothelial cells.4 Binding to specific receptors or adhesion molecules on endothelial cells is necessary for neutrophils to migrate into the extravascular space.2,3 For a brain abscess to develop, several factors must be considered, such as the virulence of the organism, the extent and duration of the bacteremia, and whether previous emboli have occurred.1 If the patient is immunocompromised, the nature of the immune defect will influence the causative organism for brain abscess. When cell-mediated immunity is abnormal the organisms responsible for brain abscess are Toxoplasma gondii, Nocardia asteroides, Cryptococcus neoformans, Listeria monocytogenes, and Mycobacterium species.1 Neutropenia or neutrophil defects can result in infections due to aerobic gram-negative organisms, Aspergillus fumigatus, Candida albicans, and the Mucorales.1 Even though the meningitis syndrome was first recognized by Hippocrates, it was not until the advent of antibiotics in the1930s that this disease process could be treated successfully. Within the United States there are 25,000 cases of bacterial meningitis annually, with 70% seen in children less than 5 years old.1 Over the past 10 years the causative agents for bacterial meningitis in infants have changed considerably with the advent of the Hemophilus influenzae type b protein-polysaccharide conjugate vaccine.5 Widespread immunization has reduced the total number of annual cases of H. influenzae type b disease in the United States by 55% and the number of cases of H. influenzae meningitis by 94%.5 The leading causes of meningitis now are Streptococcus pneumoniae (47%), Neisseria meningitidis (25%), and Listeria monocytogenes (8%). The distribution of infection from these organisms is comparable to that currently seen in the Netherlands.7 The highest mortality rate for bacterial meningitis of 26.3% is due to S. pneumoniae.8 Risk factors associated with an adverse outcome or death are age >60 years, hypotension, seizure within 24 hours of presentation, and obtundation on admission.6,8 Postoperative meningitis after craniotomy is uncommon because of the widespread practice of using prophylactic antibiotics and occurs in 1% to 6% of cases.1 Open, depressed skull fractures carry a 4% to 10% incidence of infection that can be lowered with surgical debridement.1 The infectious agents that are responsible for causing meningitis are dependent on the age of the patient. Table 11-1 lists the most common organisms causing acute bacterial meningitis by age. Gram-positive organisms that cause meningitis are S. pneumonia, which is seen in pairs, and the bacillus L. monocytogenes. Gram-negative organisms include the diplococcus N. meningitidis and the small, pleomorphic bacillus H. influenzae. Post-neurosurgical meningitis is usually caused by Staphylococcus aureus although other common gram negative pathogens are Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, particularly with ventricular drainage. Meningitis in the presence of an indwelling CSF shunt is usually due to Staphylococcus epidermidis or Propionibacterium acnes. Penetrating head trauma allows anaerobic and gram-negative organisms to access the brain, and basilar skull fractures with a concomitant CSF leak allow nasopharyngeal flora to enter the CNS. Meningitis on a chronic basis can be due to bacteria Treponema pallidum, Mycobacterium tuberculosis, and Borrelia burgdorferi (Lyme disease), the fungi C. neoformans, Histoplasma capsulatum, Blastomyces dermatitides, and Coccidioides immitis, and the parasite Taenia solium.1
Immune Response
Meningitis
| Age | Bacteria |
| Neonates (First month) | Escherichia coli |
| Gram-negative bacilli | |
| Listeria monocytogenes | |
| Streptococcus agalactiae | |
| Infants (Months 2 and 3) | Streptococcus agalactiae |
| Streptococcus pneumoniae | |
| Hemophilus influenzae type b | |
| Neisseria meningitidis | |
| Listeria monocytogenes | |
| Children (3 months to 18 years) | Hemophilus influenzae type b |
| Streptococcus pneumoniae | |
| Neisseria meningitidis | |
| Adults (18 to 50 years) | Streptococcus pneumoniae |
| Neisseria meningitidis | |
| Elderly (>50 years) | Gram-negative bacilli |
| Hemophilus influenzae type b | |
| Streptococcus pneumoniae | |
| Listeria monocytogenes |
Bacterial meningitis represents a purulent infection located in the subarachnoid space. Histologically, neutrophils are the inflammatory cells that respond to the CNS infection and reach the subarachnoid space in an unknown manner to form an exudate over the cortex that extends into the Virchow-Robin spaces.1 Infiltration of inflammatory cells into the small blood vessels can cause occlusion and subsequent cerebral infarction. Most cases of meningitis occur without an obvious source. Bacteria found in the nasopharynx and upper respiratory tract reach the CNS hematogenously and the CSF through the choroid plexus.1 The increased susceptibility of infants to meningitis is due to their underdeveloped opsonophagocytosis defenses resulting in bacteremia. Bacteria can also enter the CNS through infected thrombi in emissary veins that occur in mastoiditis, otitis media, and sinusitis. Direct inoculation of bacteria into the CNS results from osteomyelitis of the skull, dermal sinus tracts, open myelomeningoceles, orbital cellulitis, head trauma, lumbar puncture, ventriculostomy placement, placement of a CSF diversion device (shunt), or soft-tissue infection.1,7 If CNS infection develops within a week of a neurosurgical procedure, it is most likely the result of direct bacterial inoculation at the time of surgery, whereas late infections are usually the result of hematogenous spread of organisms to either damaged tissue or an implanted foreign body. Meningitis that develops after CSF shunt placement will usually occur within 2 months of surgery.1 In patients for whom temporary CSF drainage is necessary, the risk of meningitis is 6% if the catheter is changed within 5 days, compared with 18% if the drain remains in place longer.7 The presence of a previous ventricular drain does not increase the risk of infection when the catheter is replaced.
Clinically, meningitis reaches its fulminant clinical state within 72 hours of the onset of symptoms and is characterized by fever, headaches, nausea, vomiting, meningismus, nuchal rigidity, photophobia, confusion, mental status changes, lethargy, and coma. Meningeal irritation can be seen in 50% of patients with meningitis and is manifest as a stiff neck, Hoyne’s, Kernig’s, and Brudzinskis signs.8 A focal neurological deficit is uncommon with meningitis unless there has been vascular occlusion and thrombosis due to thrombophlebitis. Cranial neuropathies involving the oculomotor (III), trochlear (IV), abducens (VI), facial (VII), and the vestibulocochlear (VIII) nerves can occur in 10% of patients with meningitis.1,8 Papilledema is uncommon (1% of cases) in meningitis and should suggest another disease process.8 Seizures occur in 25% to 30% of patients with meningitis and are usually due to S. pneumoniae.8 Infants have a much higher frequency of meningitis than other age groups; the clinical findings include failure to thrive, irritability, glucose intolerance, respiratory difficulty, a bulging fontanelle, temperature instability, and jaundice.1,8
Seizures, fever, altered consciousness, and meningismus can suggest meningitis in the postoperative neurosurgical patient. In the elderly patient with multiple medical problems, obtundation without fever may represent the development of bacterial meningitis. In head-injured patients it can be difficult to diagnose meningitis clinically because of the underlying neurologic sequelae of the trauma. In all patients with an altered mental status, meningitis should be considered a potential cause for the neurological findings until the actual reason for the clinical condition has been identified.8
In bacterial meningitis, the peripheral WBC count with a predominance of polymorphonuclear leukocytes and the erythrocyte sedimentation rate (ESR) can be elevated. Blood cultures have been reported to be positive in 50% to 75% of patients with meningitis due to common causative organisms. Computed tomography is an important diagnostic test for patients with suspected meningitis that present with a neurologic deficit or seizures. This diagnostic test should be obtained before a lumbar puncture is performed to exclude the presence of an intracranial mass lesion that could precipitate a herniation syndrome if the CSF is sampled. The incidence of brain herniation has been estimated as >1% in some studies or 6% in children and infants with bacterial meningitis.6 The CT scan and the magnetic resonance (MR) imaging scan are usually normal in patients with bacterial meningitis although enhancement can sometimes be seen in the subarachnoid space or around the brain stem.1 Other intracranial processes that can mimic bacterial meningitis, such as brain abscess, subdural empyema, encephalitis, sinus thrombosis, and a parameningeal focus, can be excluded on CT or MR imaging.1 Once the CSF is sampled, it should be sent for culture, Gram stain, glucose, protein, and cell count with differential before antibiotics are administered. The presence of a parameningeal process such as a brain abscess, cranial or spinal epidural abscess, subdural empyema, osteomyelitis, or infected dermal sinus tract can mimic pyogenic meningitis on CSF analysis.
Once the CSF is sampled, the diagnosis of bacterial meningitis requires the identification of the offending organism on the Gram stain or on culture of the fluid.1 In acute bacterial meningitis, organisms are identified on the Gram stain in 60% to 90% of patients with a specificity of >97%.1,6 The opening pressure of the CSF is usually elevated, in the range of 200 to 500 mm H2O in patients with bacterial meningitis.6 Before beginning antibiotic treatment for bacterial meningitis, the WBC count is elevated in the 1000 to 5000 cells/mm3 range.6 The CSF glucose concentration is <40 mg/dL in 50% to 60% and the CSF protein concentration is elevated in virtually all patients with bacterial meningitis.6 Cerebrospinal fluid culture results are positive in 70% to 85% of patients with bacterial meningitis who have not received antibiotics, although the results may require 48 hours of incubation.6
Latex agglutination may be a useful test for the patient who has received antibiotics when the Gram stain and culture results are negative.6 A positive limulus lysate assay suggests the presence of an endotoxin in the CSF sample. The polymerase chain reaction has a sensitivity and specificity of 91% for common meningeal pathogens.6 When trying to distinguish between viral meningitis and bacterial meningitis, CSF lactate concentrations of >4.2 mmol/L were considered to be a positive discriminative factor with a sensitivity of 96%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 97%.6 In the postoperative neurosurgical patient suspected to have meningitis, empirical antimicrobial therapy should be initiated if the CSF lactate concentration is >4.0 mmol/L, pending the return of additional test results.6
Poor patient outcomes in bacterial meningitis are associated with greater amounts of antigen or a larger number of organisms in CSF samples that are obtained before initiating antimicrobial therapy.6 If there is no clinical improvement within 72 hours of initiating antimicrobial treatment, a repeat lumbar puncture should be performed; delayed CSF sterilization after 24 hours is a risk factor for subsequent neurologic sequelae.1,6 There are inadequate data to determine specific guidelines on how soon antibiotics should be started after the initial physician encounter.6 However, bacterial meningitis is a neurologic emergency and appropriate therapy should be started as soon as the diagnosis is considered likely.6 Minimum inhibitory concentrations and minimum bactericidal concentrations (MBC) should be obtained on CSF samples to ensure that the antibiotic concentration in the CSF is 10 to 20 times higher that the MBC of the offending organism.1 Factors that influence CSF drug penetration include molecular size, degree of meningeal inflammation, and lipid solubility.
Treatment for bacterial meningitis should also include those complications that can be associated with the condition, such as sepsis, seizures, shock, disseminated intravascular coagulation, and increased intracranial pressure that can lead to cerebral herniation. Anticonvulsants should be administered in the presence of seizure activity, and intravascular volume replacement should be performed in the presence of shock, using central venous pressure and pulmonary artery wedge pressure measurements for guidance. Hyponatremia, which can aggravate cerebral edema, should be avoided, and elevated intracranial pressure can be treated with diuretics, osmotic agents, and corticosteroids. The role of corticosteroids in bacterial meningitis remains controversial. However, based on available evidence, dexamethasone (0.15 mg/kg every 6 hours for 2 to 4 days) should be administered 10 to 20 minutes before, or at least concomitant with administration of antibiotics in infants and children with H. influenzae type b meningitis, and in adults suspected or proven to have pneumococcal meningitis.6 Dexamethasone decreases cerebral edema, the incidence of sensorineural hearing loss, the mortality rate for bacterial meningitis, and the production of tumor necrosis factor and interleukin-1.8
The choice of antibiotic used to treat meningitis should be based on organism sensitivity. Factors that influence the choice of antibiotic are the age of the patient and whether the infection was community-acquired or acquired in the hospital. The primary and alternative therapies for specific pathogens that cause CNS infections are listed in Table 11-2. The duration of therapy for meningitis varies, depending on the responsible organism. The duration of treatment for H. influenzae should be 7 days, N. meningitidis 7 days, S. pneumoniae 10 to 14 days, S. agalactiae 14 to 21 days, gram-negative aerobic bacilli 21 days, and L. monocytogenes ≥21 days.1,6
| Organism | Primary Therapy | Alternative Therapy |
| Bacteria | ||
| Hemophilus influenzae type b | third-generation cephalosporina | chloramphenicol, cefepime, meropenem, fluoroquinoloneb |
| Neisseria meningitidis | third-generation cephalosporina | penicillin G, ampicillin, chloramphenicol, fluoroquinoloneb, aztreonam |
| Streptococcus pneumoniae | vancomycin and a third-generation cephalosporina | meropenem, fluoroquinoloneb |
| Streptococcus agalactiae | ampicillin or penicillin G | third-generation cephalosporina |
| Streptococcus milleri | penicillin G | Erythromycin |
| Staphylococcus aureus (methicillin-sensitive) | nafcillin or oxacillin | Vancomycin |
| Staphylococcus aureus (methicillin-resistant) | vancomycin | trimethoprim-sulfamethazole |
| Staphylococcus epidermidis | vancomycin | rifampin and trimethoprim-sulfamethazole |
| Gram-negative bacilli | third-generation cephalosporina | cefepime, meropenem, aztreonam, fluoroquinoloneb, trimethoprim-sulfamethazole |
| Pseudomonas aeruginosa | ceftaziadime | piperacillin, ticarcillin and tobramycin |
| Listeria monocytogenes | ampicillin or penicillin G | trimethoprim-sulfamethoxazole |
| Nocardia asteroides | trimethoprim-sulfamethoxazole | Minocycline |
| Anaerobes | penicillin G | Chloramphenicol |
| Bacteroides fragilis | metronidazole | Clindamycin |
| Mycobacterium tuberculosis | isoniazid, rifampin, ethambutol, and pyrazinamide | |
| Treponema pallidum | penicillin G | third-generation cephalosporina |
| Borrelia burgdorferi | third-generation cephalosporina or doxycycline | penicillin G |
| Fungi | ||
| Aspergillus fumigatus | amphotericin B | Voriconazole |
| Cryptococcus neoformans | amphotericin B and flucytosine | Fluconazole |
| Candida albicans | amphotericin B or fluconazole or caspofungin | Voriconazole |
| Parasites | ||
| Naegleria fowleri | amphotericin B | |
| Taenia solium | praziquantel | Niclosamide |
| Toxoplasma gondii | pyrimethamine and sulfadiazine | Clindamycin |
a cefotaxime, ceftriaxone.
b ciprofloxacin, ofloxacin, lomefloxacin, enoxacin, pefloxacin, levofloxacin, gatifloxacin, mofloxacin.
Despite appropriate antimicrobial treatment for meningitis, 10% to 50% of patients will have permanent neurologic sequelae. Acute complications of meningitis are cerebral edema, inappropriate antidiuretic syndrome (30% of children), and ventriculitis (30%).1 Intermediate complications of meningitis include subdural empyema (1%), brain abscess, cranial epidural abscess, and hydrocephalus. Long-term complications of meningitis are learning disabilities (25% of children), motor deficits, and deafness (5% to 25% of neonates with S. pneumoniae meningitis).1 The mortality rate for bacterial meningitis is less than 10% with appropriate antibiotic treatment. For posttraumatic meningitis the mortality rate is 6%.1
Bacterial meningitis is the most common CNS infection after head injury, with the incidence ranging from 0% to 22%. The rate of meningitis when otorrhea or rhinorrhea is present ranges from 7% to 50%, in contrast to a rate of 0% to 2% for basilar skull fractures. The peak incidence for posttraumatic meningitis to develop after a CSF leak is within the first 2 weeks. Pneumococcus is responsible for the posttraumatic meningitis in 56% to 80% of patients, although the CSF culture can be sterile in up to 30%.1
Eighty-five percent of posttraumatic CSF leaks will close spontaneously within 1 week, with the majority of those remaining closing within 4 to 6 weeks.7 Those CSF leaks that do not close can be treated with continuous lumbar drainage for 5 to 7 days. Surgical exploration should be considered when the CSF drainage does not decrease within 2 weeks, persists for 6 weeks, is associated with meningitis, or is recurrent.1 Immediate surgical treatment is necessary for open dural wounds, for which the risk of meningitis after repair ranges from 1% to 10%.1
The use of prophylactic antibiotics for basilar skull fractures without meningitis is controversial and does not decrease the rate of infection. Once meningitis develops, appropriate antibiotic therapy should be directed at the responsible organism or at sinus bacteria. The main reason to withhold antibiotic treatment in the absence of meningitis is to prevent the development of resistant organisms that could lead to CNS superinfection. Antibiotics should be used in an open myelomeningocele defect where closure is delayed. A persistent dermal tract in spina bifida occulta associated with recurrent meningitis should be treated with appropriate antibiotic therapy and surgical excision.
Epidural Abscess
Approximately 2% of localized intracranial infections are due to cranial epidural abscess.10 This infection is less common than subdural empyema or brain abscess, and is usually seen in older children between the ages of 12 and 16 years.11 The location of this infection in the potential space between the inner table of the skull and the dura mater develops by direct extension, hematogenously, or by way of emissary veins. The most common location for cranial epidural abscess is adjacent to the frontal sinus; when this abscess is associated with osteomyelitis, which can be present in 25% of cases, it is known as Pott’s puffy tumor.1 If the infection erodes through the dura mater into the subdural space, an empyema will result; however, this process will rarely lead to meningitis or brain abscess. Infratentorial empyema has been found to represent 16% of cases.10
Underlying conditions that predispose to cranial epidural abscess development are frontal sinusitis, paranasal sinusitis, orbital cellulitis, rhinocerebral mucomycosis, traumatic skull fracture, mastoiditis, chronic otitis media, insertion of skull tongs for cervical traction, a congenital dermal sinus tract, and neurosurgery.1 The most common organisms responsible for cranial epidural abscess are microaerophilic or hemolytic streptococci from the sinuses, although anaerobes should be considered. After trauma or neurosurgery, S. epidermidis and S. aureus can cause cranial epidural abscess.
Clinical findings associated with cranial epidural abscess include fever, stiff neck, periorbital swelling, nausea, vomiting, headache, and lethargy, although herniation and coma have been reported with enlargement, and rapid neurologic deterioration can occur with extension into the subdural space.10,11 Scalp tenderness or a subgaleal collection have been reported with cranial epidural abscess. As with other intracranial mass lesions, lumbar puncture is contraindicated; however, if the CSF is sampled, the indices are usually unremarkable and the culture sterile.
The imaging studies of choice for cranial epidural abscess are CT and MR imaging although skull radiographs may show evidence of osteomyelitis. On CT, the abscess will have a hypodense center with peripheral contract enhancement. Magnetic resonance imaging has the advantage over CT that it can display small infectious collections in three dimensions, allowing for earlier detection. These infectious collections are usually hyperintense on T1- and T2-weighted images compared with CSF.1
Surgical evacuation of the purulent collection followed by antibiotic coverage for the infectious source identified on culture is the treatment of choice. In some patients, it is necessary to drain their infected paranasal sinuses simultaneously.10 Until the offending organism is identified, a third-generation cephalosporin should be administered and antistaphylococcal coverage should be added if a cranial defect is present. Aerobic streptococci, staphylococci, and anaerobes are responsible for the infection in 60% to 90% of patients.12 Antibiotic treatment should be continued for 6 weeks. Occasionally, antibiotic treatment alone is sufficient to cure a small infectious burden.10,13,14
Burr hole evacuation of epidural pus is usually inadequate and a craniotomy or craniectomy for drainage, with débridement and antibiotic irrigation, is essential to eradicate the infection.1 Subdural exploration or the placement of a temporary drainage catheter in the epidural space is unnecessary. Epidural abscesses that develop after neurosurgical procedures can be treated by a suction irrigation method that is successful in salvaging the bone flap in 50% of cases.1 If it is necessary to replace the bone flap, the infection should be resolved for at least 3 months before reimplantation. The morbidity and mortality (1.2%) is very low for cranial epidural abscess.10
Subdural Empyema
The association of subdural empyema with frontal sinusitis was first recognized in the 1940s.1 Between 12% and 25% of intracranial infections are due to subdural empyema which can occur after trauma in 3% and, after neurosurgery, in 4% of patients.1,15 Two thirds of patients are 10 to 40 years of age, males being affected two to three times as often as females.1,15 The growth of the frontal sinus during puberty has been offered as an explanation for the age and sex distribution.1
Otogenic and paranasal sinus disease, shunt placement, trauma, and neurosurgery are all predisposing conditions for subdural empyema. Purulence can reach the subdural space through the posterior wall of the frontal sinus, by retrograde thrombophlebitis of mucosal veins in the frontal sinus that communicate with dural venous sinuses through emissary veins, or by hematogenous spread.1 Two thirds of subdural empyemas originate from the frontal or ethmoid sinuses and 15% to 20% are the result of inner ear infections.1 Meningitis is an important predisposing cause for subdural empyema in infants.11
The causative organisms for subdural empyema are those associated with the source of the infection. Aerobic and anaerobic streptococci, particularly S. milleri, are a common source of infection from the paranasal and otogenic sinuses. After neurosurgery, gram-negative bacilli and staphylococci are common causative agents for subdural empyema. In infants, S. pneumoniae, H. influenzae, and E. coli meningitis will progress to subdural empyema in 2% of cases.1 In up to one third of cases of subdural empyema the culture results are sterile, suggesting a potential anaerobic source.
The pus can be found over the convexities, layering along the tentorium cerebelli, or in the interhemispheric fissure, where its spread is dependent on gravity, with 1% to 10% located in the posterior fossa.1,13 Cortical venous thrombosis leading to cerebral infarction has been reported in 90% of fatal cases of subdural empyema. The development of brain abscess in necrotic cerebral tissue has been reported in up to one fourth of patients.
Headache, meningismus, fever, altered mental status, nausea, vomiting, seizures, and focal neurologic deficits, most commonly a contralateral hemiparesis, are all presenting signs and symptoms of a subdural empyema in an adult.1 In infants, irritability, poor feeding, vomiting, a bulging anterior fontanelle, lethargy, stupor, and seizures can be the presenting signs and symptoms of a subdural empyema.1 The clinical signs and symptoms are often related to cortical irritation that occurs from vascular occlusion, resulting in a stroke. Once symptoms develop, the neurologic decline can be rapid although postoperative subdural empyema may present in a delayed fashion. The average duration of symptoms for a subdural empyema is 2 weeks, although the range for symptomatology is 1 to 8 weeks.1 Hydrocephalus has been reported in infratentorial empyema and requires aggressive treatment.13 The mortality rate in infratentorial empyema is high (>20%), and in all cases was due to subdural empyema.13
As with most intracranial infections, the peripheral WBC count can be elevated with subdural empyema and the blood cultures can be positive. Lumbar puncture should be avoided in the patient with a subdural empyema to prevent cerebral herniation; however, if one is done, the opening pressure is usually elevated with an increased protein level, and if meningitis is present, the culture results will be positive. Plain skull films are rarely obtained but can show sinusitis, mastoiditis, or osteomyelitis. The most revealing tests for subdural empyema are contrast-enhanced CT and MR imaging. Subdural empyemas appear as diffuse, hypodense collections that enhance peripherally but may not be apparent when they are adjacent to the falx or in the early stages of the infection. On MR imaging, subdural empyemas will display a decreased signal on T1-weighted MR imaging and an increased signal on T2-weighted scans. Magnetic resonance imaging has six distinct advantages over CT in demonstrating subdural empyemas: (1) more precise three-dimensional localization; (2) the absence of bony artifact; (3) the ability to distinguish noninfected subdural effusions; (4) an increased sensitivity for early detection; (5) an increased specificity for distinguishing subdural infection from epidural infection; and (6) the advantage of using a paramagnetic contrast agent.1,17
The appropriate management for subdural empyema combines surgical drainage with antibiotic therapy. Most patients (96%) have surgery of some type and it is important simultaneously to eradicate the source of the infection.15 In a large series of 699 patients, it was determined that craniotomy with the evacuation of pus was the preferred procedure for subdural empyema because of improved clinical outcomes and lower reoperation rates and morbidity than limited procedures such as burr holes or craniectomies.16 These limited procedures were usually reserved for critically ill patients in septic shock, for patients with parafalcine empyemas, and for children where the empyema was due to meningitis.1,16 Burr hole aspiration does allow for the instillation of antibiotics and may need to be repeated or even followed by a craniotomy in up to 20%.1 In infants with parafalcine pus, aspiration through the anterior fontanelle has proven to be an effective technique. Once the responsible organism has been identified, appropriate antibiotic treatment should be continued for at least 3 weeks, with some recommending a 4-to 6-week course of treatment.1 Medical management alone has been successful in some medically stable patients who respond rapidly to treatment. Additional medical management should include anticonvulsants to prevent seizures and corticosteroids or osmotic agents for those patients with life-threateningly raised intracranial pressure.
Good outcomes, in which 82% of patients achieved a Glasgow Outcomes Score of 4 or 5, have been reported with combined medical and surgical management.15 The morbidity rate for subdural empyema is 26% and the mortality rate is 12%.15 Burr hole drainage of subdural empyema has a higher mortality rate than does craniotomy, which is probably because of the fragile medical state of the patients receiving that treatment. The overall prognosis for subdural empyema is related to the extent of the infection, the level of consciousness of the patient, and whether there is a delay in diagnosis and treatment.1
Brain Abscess
The annual incidence of brain abscess in the United States is -2500 cases, and this frequency may be rising with the increasing prevalence of AIDS and organ transplantation.18 Young adult males in the first three decades of life are most likely to develop a brain abscess; in children the peak incidence is between the ages of 4 and 7 years.1 Of children who develop a brain abscess, 25% have congenital heart disease; children are 10 times more likely to develop a brain abscess because of their right-to-left shunt, which results from tetralogy of Fallot in 50%.1,18
The source or cause of brain abscess remains obscure in 10% to 37% of patients.18 The most common source of brain abscess in up to two thirds of patients is contiguous spread of infection from the paranasal sinuses, middle ear, or the mastoid air cells. Traumatic inoculation of the brain leading to abscess formation occurs in 9% of patients, and 25% of abscesses will result from hematogenous dissemination.1 Infectious sources of brain abscesses are osteomyelitis, dental abscesses, pulmonary infections, acute diverticulitis, and subacute bacterial endocarditis.1 Abscesses due to direct extension are usually singular; those resulting from hematogenous spread are often multiple. Abscesses in the frontal lobe are associated with paranasal sinusitis that enters intracranially by retrograde thrombophlebitis of diploic veins. Middle ear and mastoid infections cause temporal lobe abscesses by either direct extension or by thrombophlebitis of temporal emissary veins. Cerebellar abscesses result from direct extension after mastoiditis.
Brain abscess after a cardiac malformation results from chronic hypoxemia leading to polycythemia and increased blood viscosity, which causes vascular thrombosis, infarction, and necrosis of cerebral tissue.1 Abscesses develop in ischemic white matter adjacent to the cortex where the abscess capsule is thickest because the increased vascularity promotes deposition of collagen. Factors that affect capsule development include hypoxia, which inhibits fibroblast migration through the brain, thereby interfering with blood vessel and capsule formation.1 Brain abscess rupture into the ventricle occurs because the capsule is thinnest at the point furthest from the cerebral cortex. Posttraumatic brain abscesses develop soon after the injury because of contaminated debris and retained bone fragments. Postoperative brain abscess usually manifests itself in a delayed manner in less than 2% of patients and is responsible for 10% of postoperative infections following clean neurosurgical cases; meningitis makes up the remaining 90% of infections.1
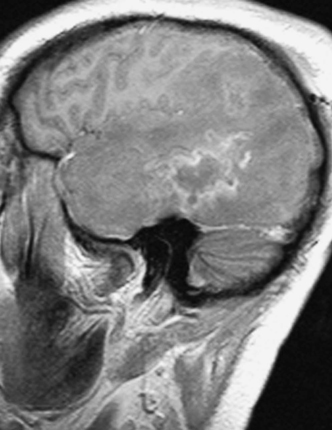
Anaerobic organisms such as streptococci and Bacteroides species. are those most often responsible for brain abscess formation, usually of otogenic or dental origin. Aerobic isolates are usually streptococci, gram-negative bacilli, and staphylococci, S. aureus being the most commonly identified organism after trauma. Organisms isolated from the sinuses causing brain abscess are aerobic streptococci, anaerobes, and H. influenzae. Fungal brain abscesses originating in the lungs of immunocompromised patients are often due to Aspergillus species. (Fig. 11-1). Recently, brain abscesses due to Toxoplasma gondii have become a common finding in patients with AIDS (Fig. 11-2). Gram-negative organisms such as E. coli, Proteus species. and Citrobacter species are a common source of brain abscess after meningitis in neonates and infants; this relates to a deficiency of placentally transferred immunoglobulins and complement.18 The majority of brain abscesses are due to a single organism, although 30% to 60% of abscesses can be polymicrobial.1
The signs and symptoms of brain abscess are those of an intracranial mass lesion, but progress much more rapidly than those seen with a neoplasm. Headache of less that 2 weeks’ duration is the most common symptom in 75% of patients. Low-grade fever is present in more than 50% of patients with brain abscess, and seizures, nausea, and vomiting, due to increased intracranial pressure, can be seen in up to 50%.1 More than 60% of patients will have a focal neurological deficit or an altered level of consciousness, ranging from confusion to coma. Nystagmus and ataxia can be seen with a cerebellar abscess from an otic source. In infants, irritability, an increasing head size, seizures, and failure to thrive can be observed with brain abscess.