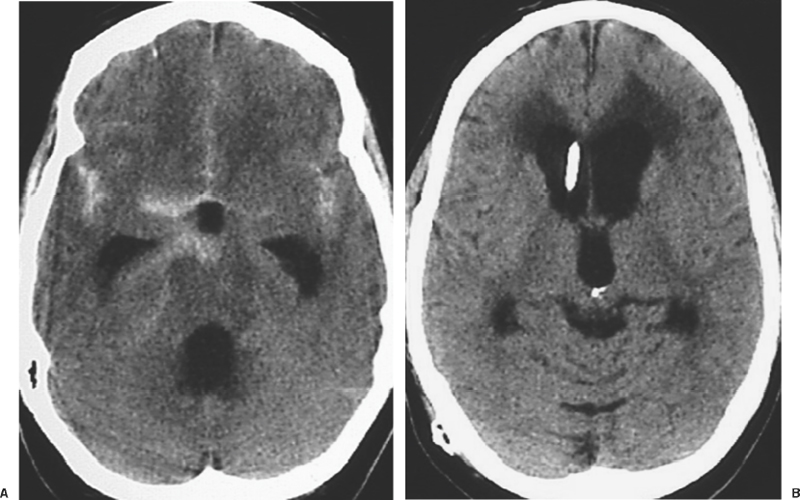
9 Acute Management of Subarachnoid Hemorrhage Pascal Jabbour and Issam Awad The annual incidence rate of subarachnoid hemorrhage (SAH) is estimated to be 10 cases per 100,000 people.1–5 The patients who make it to a hospital and receive adequate medical attention will have a higher chance of surviving, but still a large number of them will succumb to re-bleeding, to the sequelae of hemorrhage, to vasospasm, or to different medical complications.6–9 Many of the catastrophic sequelae of SAH occur in the first hours or days following the event. Prompt diagnosis and careful management in this early stage can greatly impact on the overall outcome of these patients. Conversely, delayed diagnosis or negligence of one or more management principles may result in devastating and irreversible consequences.6,10,11 Despite the widespread availability of modern diagnostic and treatment modalities, many patients do not reach specialized centers until hours or days following hemorrhage. Even in large metropolitan areas, delayed diagnosis and transfer to a center capable of definitive treatment of the problem are still prevalent, denying many patients the advantages of optimized treatment in the acute phase.6–8 There continues to be a lack of general awareness among primary and community physicians about the optimal diagnostic and therapeutic maneuvers in patients with suspected SAH. Neurosurgeons must be involved in the education of community and emergency room physicians, and in campaigns of public awareness about this entity. Few conditions in neurosurgery merit as intense and careful a diagnostic and therapeutic approach in the acute stage as SAH. Concurrent steps are taken in each case so as to arrive at optimal diagnosis, systemic stabilization, and management of neurologic sequelae. These measures are taken while planning as early as possible definitive treatment of the cause of SAH in the individual patient, so as to prevent the devastating consequences of rebleeding. A recognition of the common signs and symptoms of SAH is essential for rousing clinical suspicion and eventual diagnosis.12 Patients most commonly report a sudden onset of severe excruciating headache, typically “the worst of one’s life.” This may occur at any time, and during activity or rest, but is most frequently reported during intense activity, heavy straining, or sexual intercourse. The headache is frequently described as retro-orbital and often radiates to the nuchal area. In patients who are frequent headache sufferers, the headache induced by SAH is often different from one’s more regular headaches and clearly more intense. Patients frequently report a clustering of more minor headache complaints-labeled sentinel headaches-in the days or weeks preceding SAH. Such warning headaches may be caused by minor leak, by changes in the size of the aneurysm, and/or by mass effect on nearby structures before catastrophic rupture. Within seconds or minutes of the intense headache, the patient may lose consciousness, suffer a seizure, or die. Other patients may have persistent severe debilitating headache in subsequent hours, or a less bothersome dull and nagging discomfort. Retro-orbital pain, photophobia, nuchal discomfort, and meningeal signs persist for hours and days following SAH. In cases where these initial symptoms are misinterpreted, a variety of delayed sequelae may set in prior to definitive diagnosis. Delayed ischemic neurologic deficits, persistent unexplained “meningitis” or hydrocephalus should raise the question of the possibility of SAH in the preceding days or weeks. In some cases, sub-hyaloid retinal hemorrhages may raise questions about or increase suspicion of SAH in situations where the clinical scenario is not otherwise clear. Similarly, a wide variety of focal neurologic deficits may accompany the rupture of aneurysms in various brain locations and may enhance clinical suspicion. The diagnosis of an SAH must be executed rapidly because of the dire consequences of rebleed or misdiagnosis. It is usually done with a simple nonenhanced brain CT scan which can detect the SAH in as many as 95% of cases13,14 (Fig. 9-1). The amount of SAH is evaluated by the Fischer grading system which has a prognostic value in predicting the risk of vasospasm and overall patient outcome (Table 9-1). The CT scan may also provide useful localizing information about the possible source of hemorrhage. In instances where the CT scan is negative or questionable and where there is any clinical suspicion of SAH, a spinal tap must be performed. The presence of red cells on the acute tap would confirm clinical suspicion of SAH. The differential diagnosis of sanguinous CSF includes the possibility of a traumatic tap.12,15,16 This cannot always be distinguished with certainty from SAH. A traumatic tap is more likely to clear with decreasing red cell counts in subsequent serially collected tubes. In SAH, serially collected tubes will likely have a stable red cell count, without such evidence of clearing. A repeated spinal tap at another level, or several hours later, may assist in clarifying the situation. Figure 9-1 CT scan showing polycisternal subarachnoid hemorrhage. The etiology is most likely aneurysmal. Xanthochromia of CSF occurs due to lysis and degradation of red blood cells and hemoglobin, starts 12 to 24 hours following SAH, and may persist for several days. The presence of xanthochromia color in spun CSF is consistent with SAH, while a traumatic tap will usually result in clear supernatant following spinning of the sample. A cellular polymorphonuclear meningeal reaction may occur in the first hours postbleed, and becomes gradually more monocytic in subsequent days. This response and an accompanying elevated protein may persist in CSF for 2 to 3 weeks following SAH, and may be present even after the disappearance of xanthochromia and red cells. In the setting of negative or uncertain interpretation of the CT scan, a crystal clear CSF on spinal tap will safely and reliably exclude the possibility of significant SAH. A low threshold for performing a spinal tap should be adopted in emergency room and community physician settings whenever there is any suspicion of possible SAH. The potential consequences of misdiagnosis cannot be justified by the reluctance of patient or physician to perform a spinal tap.
Diagnosis of Subarachnoid Hemorrhage
Establishing the Diagnosis
| Fisher Group | Blood on CT |
| 1 | No subarachnoid blood detected |
| 2 | Diffuse or vertical layers ≤1 mm thick |
| 3 | Localized clot and/or vertical layer >1 mm |
| 4 | Intracerebral or intraventricular clot with diffuse or no SAH |
SAH, subarachnoid hemorrhage.
Establishing the Etiology
The prevalence of cerebral aneurysms in the population is estimated to range between 0.2% and 7.9%, with greater prevalence in older patients.17 This etiology is considered to be responsible for 70% to 80% of spontaneous SAHs. Aneurysms are known to develop at vessel bifurcations, points of maximum hemodynamic stress. The ones associated with infection or trauma tend to occur more distally in the circulation. Eighty to 90% of aneurysms affect the anterior (or carotid) circulation, at the anterior communicating artery, posterior communicating artery, middle cerebral artery, and other locations. Ten to 20% of aneurysms affect the posterior (or vertebrobasilar) circulation, most likely at the basilar summit, at the posterior inferior cerebellar arteries, and at other locations. Aneurysms can be classified by shape, with the great majority of aneurysms saccular or berry-shaped, involving an eccentric pathology of the arterial wall, usually at a branching point. A small fraction of aneurysms are fusiform, with or without saccular protrusions, reflecting more diffuse vessel wall pathology, including arteriopathy, dissection, or infection. Saccular aneurysms are classified by size: small, if less than 10 mm in diameter (78%); large, from 10 to 24 mm in diameter (20%); and giant, if more than 24 mm in diameter (2%).
The pathogenesis of saccular aneurysms is not fully understood, although their risk factors appear to be both congenital and acquired. Some systemic conditions are associated with the presence of cerebral aneurysms. These include connective tissue disorders (including Ehlers-Danlos syndrome, Marfan syndrome), autosomal dominant polycystic kidney disease, fibromuscular dysplasia, and atherosclerosis, but these account for only a small fraction of all aneurysms. Approximately 20% of patients with aneurysms have a family history of aneurysm affecting a first-degree blood relative.18 Hypertension and smoking appear to contribute to the risk of aneurysm formation, and also to the risk of hemorrhage.19 Risk of hemorrhage increases with larger aneurysm size.20 The annual risk of hemorrhage for unruptured aneurysms varies between 0.1% and 5% to 10% per year, with highest risks in giant aneurysms. Higher bleed risk also occurs in patients who have bled from another aneurysm, and in aneurysms at certain locations (basilar summit and anterior communicating arteries).21,22 Twenty percent of patients harbor multiple aneurysms.
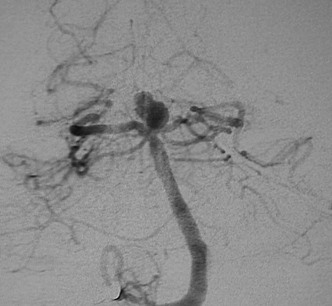
Figure 9-2 Angiogram revealing basilar summit region aneurysm.
Once diagnosis of SAH is established, a 4-vessel cerebral angiogram is the most sensitive and specific modality for diagnosis of aneurysm, and this will typically reveal the etiology of the bleed (Fig. 9-2). Modern angiographic protocols may include digital subtraction technique and recently available rotational 3-dimensional assessment of the aneurysm (Fig. 9-3). These vastly enhance image quality and provide enhanced information for therapeutic planning.
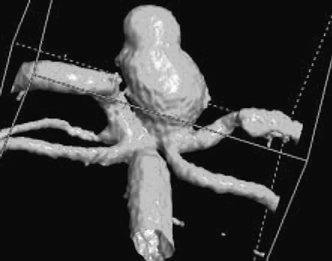
Figure 9-3 Three-dimensional (3D) rotational angiogram in same case depicted in Figure 9-2, revealing much more spatial resolution than in the conventional angiogram images. The information in 3D angiography can help guide therapeutic decisions regarding endovascular versus surgical intervention.
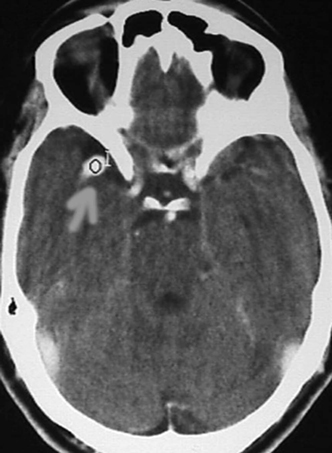
Figure 9-4 CT scan with contrast, showing middle cerebral artery aneurysm.
In cases where the angiogram fails to show the etiology, magnetic resonance imaging (MRI) should be done to rule out any angiographically occult lesion as a source of hemorrhage. Then a repeat angiogram should be performed 1 to 2 weeks after the first study. A CT scan with contrast, magnetic resonance angiogram (MRA), or computed tomographic angiogram (CTA) may establish diagnosis of aneurysm (Fig. 9-4 to Fig. 9-6) with less invasiveness, but also with less sensitivity than angiography. These tests are used in cases where diagnosis of SAH is questionable or where risks, the patient’s medical condition, or immediate availability of angiographic facility or personnel may preclude emergent angiography.
Perimesencephalic SAH is more likely to be associated with negative angiography, and no specific etiology of SAH is often found in that setting (Fig. 9-7). However, this must remain a diagnosis of exclusion, as basilar or other posterior circulation aneurysms may also cause perimesencephalic bleeds.
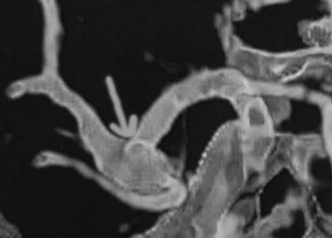
Figure 9-5 CT angiogram, performed by computer reconstruction of thin-cut high-resolution CT scan with contrast, reveals an aneurysmal dilatation at the middle cerebral artery.
In arterial dissection the cerebral angiogram may reveal one of the following findings: luminal stenosis, complete occlusion, double lumen sign, fusiform dilatation, frank extravasation of dye, or pseudoaneurysm (Fig. 9-8). An arterial dissection may be associated with normal luminal filling on angiography, and is not excluded by a negative angiogram. The MRI scan, with axial T1 sequences and MRA source images, is more sensitive than catheter angiography (and the reconstructed MRA) for the diagnosis of an arterial dissection. They reveal a crescent sign that is the hematoma in the vessel wall as a bright signal surrounding the signal void of the carotid or vertebrobasilar arteries on axial T1-weighted or source images. MRI also provides assessment of thrombosed portions of aneurysms, as in giant lesions, that may not fill on the angiogram (Fig. 9-9).
If angiography does not reveal the source of SAH, a systematic search of other causes is undertaken, including an MRI of the brain and spine, performed with and without contrast and with dissection detection protocol. This would reveal occult vascular malformations, dissections, or tumors. If none is found, a repeat cerebral angiogram is performed, a week or more later, this time with external carotid selective injections in addition to traditional 4-vessel views, to exclude dural fistulae. A second angiogram is not performed if another etiology of SAH is found or if the bleed was solely limited to perimesencephalic cisterns and the first angiogram was of excellent quality.
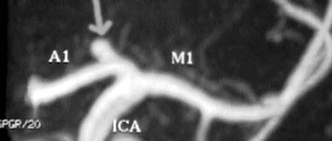
Figure 9-6 Magnetic resonance angiogram (MRA) revealing a carotid summit berry aneurysm. This is an excellent modality for screening patients for aneurysms. A negative MRA is not sufficient to exclude aneurysm in a patient with subarachnoid hemorrhage.
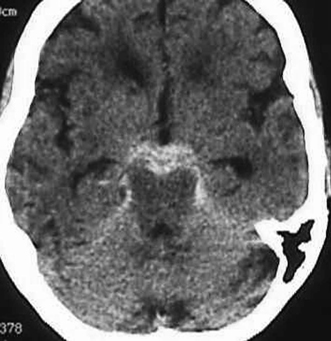
Figure 9-7 Perimesencephalic SAH. Cerebral angiogram did not reveal an aneurysm. A perimesencephalic SAH may also be caused by basilar aneurysm or other etiologies. SAH, subarachnoid hemorrhage.
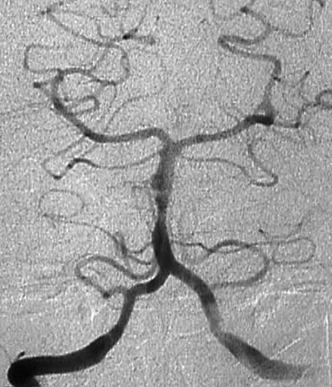
Figure 9-8 Angiogram of patient with severe subarachnoid hemorrhage, revealing dissection of the vertebral artery, with double lumen and small aneurysmal dilatation.
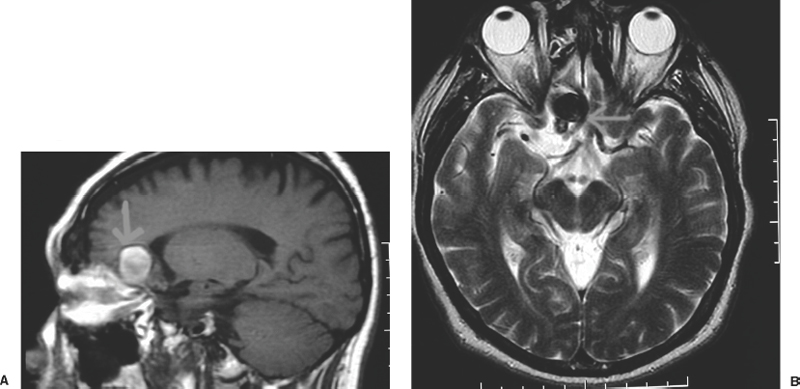
Figure 9-9 Magnetic resonance imaging of partially thrombosed giant aneurysm. T1-weighted (A) and T2-weighted (B) imaging.
Grading Subarachnoid Hemorrhage
The patient’s level of consciousness is a cardinal determinant of outcome after SAH, and it can affect treatment decisions as well as prognostication. It can be assessed using the Hunt and Hess grade (Table 9-2), or the World Federation of Neurological Surgeons grade (Table 9-3). The former is quite simple and widely used, while the latter grade has been shown to have better positive and negative predictive power in relation to outcome, especially among high-grade patients.19,23,24
| Grade | Neurologic Status |
| I | Asymptomatic; or minimal headache and slight nuchal rigidity |
| II | Moderate to severe headache; nuchal rigidity; no neurologic deficit except cranial nerve palsy |
| III | Drowsy; minimal neurologic deficit |
| IV | Stuporous; moderate to severe hemiparesis; possibly early decerebrate rigidity and vegetative disturbances |
| V | Deep coma; decerebrate rigidity; moribund appearance |
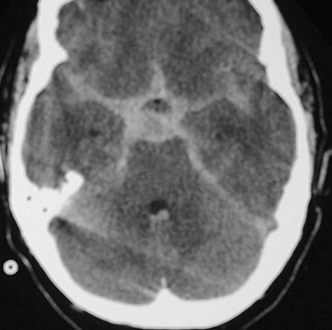
Cases with cranial neuropathy may represent elevated intracranial pressure (ICP-abducens or oculomotor palsies), or aneurysmal compression on the cranial nerve (posterior communicating artery or superior cerebellar artery aneurysm compression on the oculomotor nerve). Other focal neurologic deficits likely imply an intracerebral hemorrhage (ICH) in addition to the SAH, as is common with middle cerebral artery aneurysms (Fig. 9-10). Vascular malformations and fistulae are more likely to cause ICH than SAH, but could result in both. Aneurysmal bleeds from anterior communicating artery and basilar summit, or from posterior inferior cerebellar artery aneurysms, may cause intraventricular hemorrhage, and this in turn may cause ventricular obstruction and account for decreased level of consciousness.
| WFNS grade | Glasgow Coma Scale Score | Major focal deficit |
| 0 (intact aneurysm) | — | — |
| 1 | 15 | Absent |
| 2 | 13–14 | Absent |
| 3 | 13–14 | Present |
| 4 | 7–12 | Present or absent |
| 5 | 3–6 | Present or absent |
WFNS, world federation of neurological surgeons.
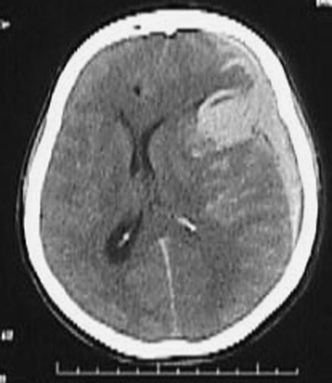
Figure 9-10 CT scan in comatose patient with massive SAH and ICH (plus subdural hemorrhage). Such a patient is taken emergently for surgical evacuation of hematoma, with or without contrast-enhanced CT scan, but without taking additional time for conventional angiography. SAH, subarachnoid hemorrhage; ICH, intracronial hemorrhage.
Systemic Stabilization
The acute resuscitation of SAH patients follows the general guidelines of the “ABC.” Any patient with a Glasgow Coma Scale (GCS) score of 8 or less, or unable to protect the airway, should be intubated, especially for planned transport. Anti-seizure prophylaxis should be administered as soon as possible because seizures can increase morbidity and brain edema. A central venous line and an arterial line should be inserted to assist with acute management.
Blood pressure should be controlled aggressively, and both hypertension and hypotension should be avoided. Hypertension should be avoided because of the presumed presence of an unsecured vascular lesion. Hypotension should be avoided because it could compromise cerebral perfusion, especially in the setting of elevated ICP in an unconscious patient.25
Coagulation parameters should be examined and corrected promptly.
The patient should be given stool softeners to avoid any physical effort that could cause rebleeding of the cerebral aneuryrsm. Pain management should be optimized, and the patient should be transferred as soon as possible to a critical care environment where these measures are maintained, along with other multisystem homeostasis, as further diagnostic and therapeutic interventions are planned.
Cardiac Complications
Subarachnoid hemorrhage has been shown to induce subendocardial ischemia, proportional to the severity of neurologic insult. Electrocardiographic changes, elevation of myocardiac enzymes, ventricular wall motion abnormalities, and life-threatening arrythmias can be seen in patients with SAH, especially in the acute phase.26,27 These do not usually alter the course of the illness, and typically should not prevent timely interventions for diagnosis or therapy. Cardiac complications can be life-threatening in the setting of pre-existing cardiopathy, or after multisystem complications of illness or therapies (i.e., myocardiac depression by barbiturates). These complications should be monitored and treated prophylactically. Beta blockers play an important role in treating the catecholamine-mediated complications.28–35
Pulmonary Complications
Patients may develop pulmonary complications for a variety of reasons. Those patients with poor neurologic grade are at increased risk of aspiration, atelectasis, pneumonia, and pulmonary embolism.29 Neurogenic pulmonary edema is a complication that occurs after significant neurologic insult, and consists of leakage of protein-rich fluid into the pulmonary alveoli. It is believed to be due to the disruption of the endothelial barrier in response to massive sympathetic discharge.36 Volume overload during hyperdynamic therapy for vasospasm can cause or exacerbate pulmonary edema. Sometimes it is important to insert a Swan-Ganz catheter and gather as much information as possible to try place the patient on the Starling curve, and to manage fluids appropriately.
Hyponatremia
Hyponatremia is common in patients with SAH.20 It may result from two mechanisms, the syndrome of inappropriate antidiuretic hormone (SIADH) with free water retention, or inappropriate natriuresis (also known as cerebral salt wasting) mediated by the atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP).37,38 Determining the likely cause is important because the two syndromes are managed differently. Hyponatremia is treated with fluid restriction in the setting of SIADH, as is common after ICH. Hyponatremia is treated with hypertonic fluid and salt replacement in natriuresis syndrome, common after SAH. The fluid balance (intake versus output), urine sodium concentrations, and the clinical setting assist in differentiating the two syndromes. Patients who suffer SAH have been shown to be predominantly fluid-contracted from inappropriate natriuresis. Especially during periods of vulnerability to vasospasm, these patients can suffer irreversible brain infarctions if they are further fluid-restricted. Volume and salt maintenance are the mainstay of therapy during vasospasm after SAH.39–43 Hypernatremia may be caused by diabetes insipidus (DI) and should be treated with free water replacement and vasopressin.119
Other Medical Problems
Patients with a myriad of associated medical conditions may sustain SAH. In general, management of SAH takes priority over the definitive treatment of these other associated conditions. Highly individualized management decisions are necessary in this regard, weighing the advantages and disadvantages of every intervention with regard to the SAH and the associated medical conditions. When possible, stabilization measures should be undertaken for all non-life-threatening conditions while treatment of SAH is undertaken. Typically, the presence of other medical conditions increases the urgency of definitive treatment of SAH, prior to addressing these other problems.
In cases in which associated life-threatening medical problems preclude definitive treatment of SAH, supportive medical and neurologic measures are undertaken until such time as definitive therapy becomes advisable.
Pregnancy
The management of a pregnant patient with SAH should take into consideration potential harm to the mother and fetus.44 Often, the mother is treated urgently as if she were not pregnant, with the added specific precautions taken so as to protect the fetus and the pregnancy. In cases of SAH in the third trimester of pregnancy (and with a viable fetus), simultaneous cesarean delivery and definitive management of the aneurysm should be undertaken. Again, decisions are highly individualized according to the particular clinical scenario, including considerations of the condition of the mother, the fetus, the status of the pregnancy, and the difficulty of proposed treatment of the cerebral aneurysm. Rebleeding and other sequelae of SAH account for a large number of maternal and fetal deaths, many of which would be preventable by careful and well-coordinated neurosurgical and obstetric care. Again, missed or delayed diagnosis can result in devastating consequences in many such cases.44–47
Drug Abuse
Several legal and illegal drugs, usually stimulants, have been associated with SAH. Cocaine abuse48,49 in various forms is increasingly encountered in this setting. A careful history must be documented in this regard, although denial is prevalent. Urgent drug screening on urine sample should be obtained upon admission. Patients who bleed following or during drug use are likely to harbor cerebral aneurysms, and this is the most likely source of hemorrhage. They are managed in an identical fashion, with added attention to potential drug overdose or subsequent withdrawal complications.
Subarachnoid hemorrhages at higher incidence have been reported in women taking certain vasoactive drugs including decongestant cold remedies and diet pills containing phenylpropranolamine.50
Neurologic Management
After medical stabilization, several measures are instituted so as to prevent and treat the early and delayed sequelae of SAH.
Prevention of Rehemorrhage and General Acute Management
The major cause of death in patients who survive an initial aneurysmal SAH is rebleeding. The timing of intervention should consider this risk, and there is a general consensus that the good-grade patients should have early intervention to eliminate the aneurysm from the circulation within the first 48 hours.51 In experienced neurovascular centers early treatment of aneurysm is also performed on poor-grade patients if they are hemodynamically stable.52
Arterial dissection that has caused an SAH also mandates early therapeutic intervention, aimed typically at excluding the dissected segment from the circulation. Other etiologies may require urgent treatment, such as correction of coagulopathy.
Subarachnoid hemorrhage must be treated as a neurosurgical emergency.
In patients with SAH the major cause of early death during the first 24 hours is rebleeding from unsecured aneurysms.19,51 Controlling blood pressure will help prevent rebleeding after aneurysmal rupture. Prevention of even brief periods of hypertension is of paramount importance in preventing rehemorrhage.7,10,11,53 A potent hypotensive agent such as nicardipine should be premixed at the bed-site to facilitate control of hypertension. In practice, the authors maintain a mean arterial blood pressure (MAP) between 70 mm Hg and 90 mm Hg.
In the case of SAH and intraventricular hemorrhage, or any hemorrhage compromising the CSF circulation pathways, acute hydrocephalus should be suspected and treated with a ventriculostomy, performed as a quick bedside procedure.
The breakdown products of subarachnoid blood are probably responsible for spasm in cerebral arteries following SAH.19,54 Vasospasm begins 3 to 5 days after the SAH and may last for 2 to 3 weeks, during which the brain is vulnerable to further ischemic insults. Nimodipine at 60 mg PO q4h for 21 days should be started on the day of admission; it has been shown to decrease neurologic sequelae from vasospasm.19,55–57 Noninvasive monitoring for vasospasm should be instituted during this vulnerability period, with other treatments instituted (hyperdynamic therapy and endovascular interventions) if there is evidence of progression or neurologic symptoms.
All patients with hemorrhagic stroke should be treated with anticonvulsants to prevent early seizures that can increase rebleeding and elevated ICP.
The breakdown of the blood-brain barrier will cause edema in the normal brain.
The cerebral perfusion pressure (CPP) is the pressure gradient responsible for cerebral blood flow, and its compromise results in cerebral ischemia. The CPP is defined as mean arterial pressure minus intracranial pressure (MAP – ICP). Monitoring of ICP can be used to guide CPP management58,59 whenever intracranial hypertension is suspected or is compromising cerebral perfusion. Elevated ICP is defined as intracranial pressure exceeding 20 mm Hg for >5 minutes. The goal for treatment of elevated ICP is ICp < 20 mm Hg and CPP >70 mm Hg.7 An ICP monitor should be placed in all patients with GCS <9 or who cannot be followed by a neurological exam. Intraparenchymal fiberoptic ICP monitors and intraventricular monitors are commonly used, the former being more accurate and less vulnerable to obstruction, while the latter allow simultaneous drainage of CSF to treat elevated ICP.
Intracranial hypertension can be treated by diverting CSF, by decreasing brain tissue bulk or cerebral blood volume, or by sedation and decreasing brain metabolic demands. External ventricular drainage allows diversion of CSF whenever ICP exceeds a certain level, and may be performed continuously (by titrating the level of the drip chamber) or intermittently depending on ICP. External ventricular drainage is ineffective if the ventricles are slit from brain edema or overdrainage, or if the catheter is obstructed by clotted blood. At the same time, efforts are directed at reducing the pressure gradient across the aneurysm wall (i.e., transmural pressure, which represents the intracranial MAP minus the ICP) and impairing dissolution of the clot that has sealed the site of previous rupture; thus overdrainage should be avoided, and drainage should reduce ICP to no lower than 15 mm HG.14
Brain bulk may be treated to lower ICP with osmotherapy, using mannitol 20% (0.25 to 0.5 g/kg every 4h) and furosemide (10 mg q2 to 8h); these are administered alternately or simultaneously as needed for ICP waves, but one should keep in mind that using diuretics in patients during the period of vasospasm can be deleterious and can increase the risk of stroke. Serum osmolarity and sodium concentrations should be measured at least twice a day to target an osm <310 mOsm/L(50), and fluid administration should aim to maintain euvolemia and normonatremia. Osmotherapy cannot be used to treat ICP if extremes of hypovolemia and hypernatremia are allowed to develop.
Hypocarbia (25 to 35 mm HG) decreases the ICP by causing a cerebral vasoconstriction, and this can be very effective in acute crises with waves of elevated ICP. Extreme hyper-ventilation (pC02 <20 mm Hg) can exacerbate brain ischemia by decreasing cerebral blood flow. Hyperventilation should also not be used for an extended period because it can become ineffective with metabolic adjustment to respiratory alkalosis. Further response to life-threatening ICP waves becomes ineffective after chronic hyperventilation, and the patient becomes vulnerable to rebound increased ICP when restoring normocapnea.60
Sedation (propofol, benzodiazepine, or morphine) with neuromuscular paralysis can reduce elevated ICP; it does so by preventing agitation and straining and by decreasing brain metabolic demands. If after maximizing medical treatment the ICP is still high, induced barbiturate coma may be instituted with continuous EEG monitoring. A central line and arterial line are used, and even a Swan-Ganz catheter and pressors, if needed to maintain hemodynamic support during barbiturate-induced coma.60 Barbiturates can decrease ICP in proportion to the level of sedation, down to EEG burst suppression. Further administration of barbiturates beyond effective EEG burst suppression offers no additional benefits of ICP control, while increasing toxic complications.
Pain and agitation should be treated with sedative agents that may be used with moderation while keeping patients alert enough to be able to follow their neurological exams.
Hydrocephalus
Hydrocephalus is documented in 15% to 20% of patients with SAH.61,62 It is explained by blood interfering with CSF circulation in the ventricles, sylvian aqueduct, or the basal cisterns.63
A ventriculostomy should be performed whenever there is ventriculomegaly on the CT scan, especially if associated with altered mental status or with intraventricular hemorrhage (Fig. 9-11). It is a bedside procedure, using a sterile technique and compact cranial access kits for twist drill or burr hole. In one study there was an improvement in 80% of the patients in whom this took place.62,64,65 Overdrainage should be avoided because it can provoke aneurysmal re-bleeding62,66,67 by rapid modification of the transmural pressure. Overdrainage may precipitate slit ventricles and prevent further CSF drainage for treatment of elevated ICP. Optimal ventricular drainage aims to keep ICP below 10 to 15 mm Hg.