CHAPTER 344 Acute Medical Management of Ischemic/Hemorrhagic Stroke
Stroke is the second most common cause of mortality in people 60 years and older worldwide.1 Each year, 5.54 million die of stroke worldwide. In the United States each year, 780,000 people have a new or recurrent stroke, and on average, every 40 seconds someone in the United States has a stroke.
Therapeutic interventions after acute stroke must be initiated early because irreversible neuronal damage can occur within minutes.2,3 The extent of ischemic injury is dependent on the specific neuronal vulnerability and residual CBF through the collateral circulation.4 Early reperfusion of the infarcted area can reduce the degree of ischemia and improve neurological outcome. Late reperfusion has been associated with increased hemorrhage inside the ischemic area (hemorrhagic conversion) and increased edema (reperfusion injury).
Currently, the only approved reperfusion therapy after ischemic stroke is tissue plasminogen activator (t-PA) administered within 3 hours of stroke onset.5 In addition, a recently published report showed improved outcome in selected patients when t-PA was administered within 4.5 hours.6 Other proven interventions after hemorrhagic and ischemic stroke include blood pressure management and supportive therapies such as glucose and temperature control.
Pathophysiology of Ischemic Stroke
Most acute focal ischemic events in the brain are due to embolism or in situ thrombosis.7 Thrombogenesis is dependent on a series of complex events that promote the formation of a stable clot. Platelet aggregation, endothelial injury, and fibrin formation are key components. Angiographic studies have revealed several preferential sites for atherothrombotic lesions. Lesions in the proximal internal carotid artery and common carotid artery bifurcation are found in 50% to 80% of ischemic stroke patients, and a cardioembolic source is suspected in approximately 15% to 20% of all ischemic strokes. Although any cardiac disease is a potential source of embolization, the clinical conditions most often associated are nonvalvular atrial fibrillation, prosthetic heart valves, acute myocardial infarction, rheumatic heart disease, and ventricular aneurysm. Hereditary or acquired hemostatic disorders associated with thrombotic conditions generally involve the venous rather than the arterial circulation and are frequently seen in young adult stroke patients (4% to 5%).
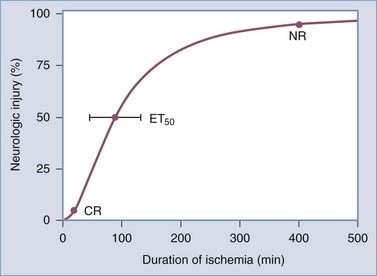
Data obtained from animal studies and clinical observations suggest that the amount of time before ischemia produces irreversible injury is relatively brief. Irreversible focal damage begins within several minutes after a significant reduction in CBF and is complete within approximately 6 hours. Figure 344-1 depicts the relationship between the duration of ischemia and the severity of the resulting neurological deficit.3 As the duration of ischemia is extended, there is a brief period during which the neurological injury is completely reversible if blood flow is restored. This type of injury is termed a transient ischemic attack. In a short amount of time, however, an ischemic event can result in irreversible injury, and as the ischemic period increases, the degree of the resulting neurological deficit increases. Ultimately, a point is reached where the injury produced becomes permanent and no intervention will result in tissue recovery.
In most cases the ischemic area is dynamic. Some regions supplied by the affected blood vessel are densely ischemic and require prompt restoration of blood flow to prevent irreversible damage. Other areas still receive some perfusion and are potentially salvageable if the cascade of cellular events resulting in neuronal death is blocked. In the past, this was simplistically viewed as an ischemic core surrounded by a region of incomplete ischemia. The latter has been referred to as the ischemic penumbra.8 The size and duration of the penumbra are unknown for any individual patient, and the topographic relationship between the ischemic core and penumbra is much more complex than initially thought based on recent preclinical and brain imaging studies.9
Pathophysiology of Hemorrhagic Stroke
Hemorrhagic stroke is caused by rupture of an intracranial blood vessel. In Western countries, 10% to 20% of strokes are caused by intracranial hemorrhage (ICH).10 Thirty-day mortality is higher than with ischemic stroke (50% versus 19%) and is affected by hematoma volume, patient age, admission Glasgow Coma Scale score, location of the hematoma above or below the tentorium, and the presence of intraventicular hematoma.11 The major risk factor for ICH is chronic arterial hypertension. Most of these hemorrhages occur in the thalamus, basal ganglia, cerebellum, or pons. In the elderly, especially those suffering from Alzheimer’s dementia, amyloid angiopathy is a frequent cause of ICH. Hemorrhage from this condition affects mostly the cortical areas. Another major cause of ICH is the use of anticoagulants. Warfarin is most often used after deep venous thrombosis or in patients with atrial fibrillation for the prevention of cardioembolic stroke.12
Patient Evaluation
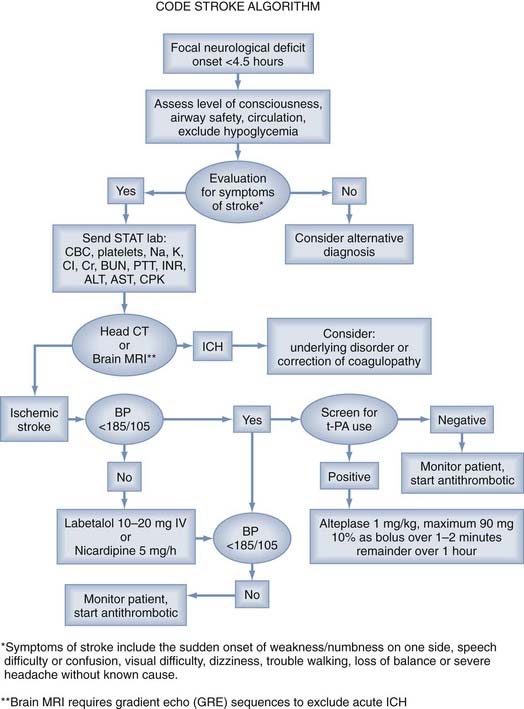
All patients with acute focal neurological symptoms should be evaluated immediately13 (Fig. 344-2). The evaluation must be brief and is similar to that for other critically ill patients. Special attention should be paid to the vital signs, including serial blood pressure measurements and level of consciousness. The history should include the time of symptom onset and any occurrence of similar neurological events, including the existence of stroke risk factors (arterial hypertension, diabetes, cardiac arrhythmia). Disease processes that can mimic stroke symptoms (migraine, hypoglycemia, postictal paralysis) should be excluded as causes if possible. In addition, the presence of serious coexisting illnesses and the recent use of oral anticoagulants should be determined.
During the brief initial evaluation, physical examination may be enhanced by the use of formal stroke scores, such as the National Institutes of Health Stroke Scale (NIHSS) (Table 344-1). The score helps ensure that the core elements of the neurological examination are assessed in timely manner, aids in communication with other health care providers, helps identify the location and extent of the neurological deficit, and provides early measures for prognosis.
Even though CT has been in routine clinical use for more than 30 years and has high sensitivity for the detection of acute ICH,14 MRI is being used increasingly frequently for the acute evaluation of stroke. Initially, MRI was inferior to CT in the detection of hyperacute blood,15 but since the introduction of gradient echo sequencing, higher sensitivity has been reported.16 Whether MRI will be as useful as CT in guiding treatment remains to be seen.
Other diagnostic tests for immediate evaluation after stroke are summarized in Table 344-2. These tests must include blood glucose, serum electrolytes, renal function, complete blood count with platelets, and coagulation studies. Markers of cardiac ischemia and an electrocardiogram should be assessed to evaluate for the possibility of myocardial infarction.
TABLE 344-2 Immediate Diagnostic Studies: Evaluation of a Patient with Suspected Acute Ischemic Stroke
CT, computed tomography; INR, international normalized ratio; ECG, electrocardiography; MRI, magnetic resonance imaging.
* Although it is desirable to know the results of these tests before giving recombinant tissue plasminogen activator, thrombolytic therapy should not be delayed while awaiting the results unless (1) there is clinical suspicion of a bleeding abnormality or thrombocytopenia, (2) the patient has received heparin or warfarin, or (3) the use of anticoagulants is not known.
From Adams HP Jr, del Zoppo G, Alberts MJ, et al. American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
Reperfusion
Intravenous Thrombolysis
Intravenous recombinant t-PA (Alteplase) administered within 3 hours of the onset of symptoms in patients with ischemic stroke is effective and a proven therapy in patients older than 18 years. Before t-PA can be used, a brief but thorough evaluation must exclude ICH, coagulopathies, low platelet count, and other factors precluding the safe use of systemic thrombolysis. To exclude ICH, all patients with acute neurological deficits should undergo (1) a detailed history and physical evaluation to establish the precise time of symptom onset or when the patient was last well, extent of the deficit, comorbid conditions, and medication use; (2) emergency laboratory evaluation; and (3) neuroimaging with cranial CT or MRI with a gradient echo sequence (Table 344-2). A reliable time of onset must be ascertained before administration of the drug. If the time of onset cannot be established or the patient awoke with symptoms, the time that the patient was last known to be well is used.
Use of t-PA within 3 hours of stroke onset is well proven to improve patient outcome. Thirty-nine percent of patients treated with t-PA had a good outcome (modified Rankin scale [mRs] score of 0 or 1) versus 26% of control patients. This is a 13% absolute treatment benefit and leads to a number needed to treat (NNT) of just 8. Pooled analysis of 2775 patients from the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS), European Cooperative Acute Stroke Study (ECASS), and National Institute of Neurological Disorders and Stroke (NINDS) trials treated with t-PA suggests a benefit up to 4.5 hours; however, the odds ratio was low (1.4; 95% confidence interval, 1.1 to 1.9), with the confidence approaching 1.0.17 A recent study by Hacke and colleagues showed that in selected patients, t-PA improves outcome when used up to 4.5 hours.6 Treating patients younger than 80 years without previous stroke and diabetes between 3 and 4.5 hours after onset led to a favorable outcome in 52.4% of all treated patients versus 45.2% of those untreated. The risk for symptomatic ICH was 2.4% versus 0.2%.6 Use of thrombolytic agents beyond 4.5 hours should be restricted to a selected few patients, and further randomized trials are needed to establish its safety and efficacy.
A large meta-analysis of 15 published studies of intravenous administration of t-PA after stroke revealed a symptomatic ICH rate of 5.2% and a favorable outcome in 37.1%.18
There are several contraindications and warnings related to the intravenous administration of t-PA. Some of the exclusion criteria include evidence of ICH on CT, elevated blood pressure (systolic >185 mm Hg or diastolic >110 mm Hg) on repeated measurements despite the administration of antihypertensive agents, and known platelet diathesis and recent use of an anticoagulant with an international normalized ratio (INR) greater than 1.4 (Table 344-3).
TABLE 344-3 Characteristics of Patients with Ischemic Stroke Who Could Be Treated with Tissue Plasminogen Activator
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|