Angiography and Endovascular Neuroradiology
Charles A. Bruno Jr
Daniel H. Sahlein
INTRODUCTION
Interventional neuroradiology (INR) or endovascular surgical neuroradiology (ESNR) encompasses all invasive diagnostic and therapeutic interventions for diseases, often neurovascular, of the central nervous system and often the skull base, neck, spinal cord, and paraspinal soft tissues. INR is a continually evolving field that has made tremendous strides over the last several decades based largely on technologic advances leading to more therapeutic options and improvements in procedure safety and efficacy, leading to increasing acceptance of endovascular therapies by the biomedical neuroscience community.
TECHNIQUE
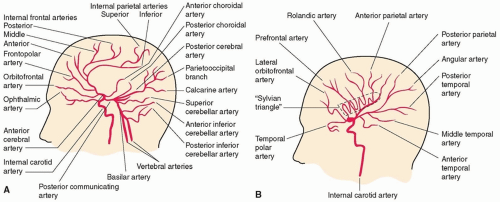
Cerebral angiography provides high-resolution images of the extracranial and intracranial cerebral vasculature (Fig. 24.1). The procedure is performed by accessing the femoral artery and threading a small catheter into the precerebral vessels.
The first cerebral angiogram in a living patient was performed by Egas Moniz in the early 1920s. During its infancy, cerebral angiography was performed via direct percutaneous puncture of the cervical carotid and vertebral arteries. This remained the principle technique for cerebral angiography until the late 1960s when the transfemoral approach was introduced. Transfemoral catheterization offered several advantages, not the least of which included access to all four cerebral vessels with one puncture, improved patient comfort, less need for general anesthesia, the ability to perform cerebral angiography on an outpatient basis, and diminished stroke risk because of limited trauma to the precerebral arterial circulation. In the modern medical era, cerebral angiography is overwhelmingly performed via the transfemoral approach.
Although advances in noninvasive imaging of the cerebral vasculature have markedly improved over the last two decades, catheter angiography remains the gold standard for imaging cerebral blood vessels. Catheter angiography has two significant advantages over noninvasive vascular imaging; improved temporal resolution, meaning images can be acquired at a rate of 10 per second versus approximately 1 per second for computed tomography (CT) and magnetic resonance imaging (MRI); and spatial resolution— the voxel size (smallest unit for which there is only one signal, akin to a pixel on a cathode ray television) for fluoroscopy on the latest equipment is 0.1 × 0.1 mm versus an average of 0.4 × 0.4 × 3 mm for CT and 1.0 × 1.0 × 0.9 mm for an average clinical MRI. Figure 24.2 demonstrates both the differences in spatial and temporal resolution between postcontrast MRI and digital subtraction catheter angiography.
Mastery of both its acquisition and interpretation serves as the foundation for all interventional procedures. Modern catheter angiography is a relatively safe procedure associated with a low complication rate when performed by neurointerventionalists even in a highly complex patient population.
CONDITIONS DIAGNOSED AND TREATED WITH ANGIOGRAPHIC TECHNIQUES
Angiography is useful for diagnosing a variety of conditions that affect the brain and spinal cord (Table 24.1). Because of its invasive nature, angiography functions as both a diagnostic and therapeutic modality. Major conditions that can be diagnosed or treated with angiographic techniques are discussed in the following sections. The most common complications of diagnostic and interventional angiographic procedures include stroke due to vessel occlusion or thrombosis and hemorrhage due to vessel perforation, dissection, and groin hematoma.
CEREBRAL ANEURYSMS
Cerebral aneurysms constitute a relatively common cerebrovascular abnormality with a prevalence of approximately 4%. Subarachnoid hemorrhage (SAH) is the most feared and common clinical presentation (see Chapter 39). Endovascular treatment of cerebral aneurysms has evolved dramatically over the past two decades and can now be offered as a viable alternative to craniotomy and microsurgical clipping in most cases.
TABLE 24.1 Conditions Diagnosed by Angiography | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
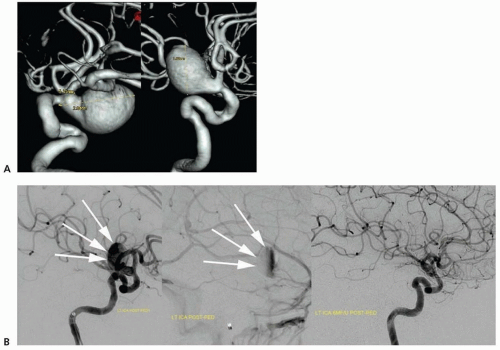
Early endovascular endeavors to treat intracranial aneurysms used detachable balloons, pushable microcoils, and liquid embolic agents with varying degrees of procedural efficacy and safety. In 1991, Guido Guglielmi revolutionized endovascular treatment of cerebral aneurysms with the Guglielmi detachable coil (GDC). The GDC is designed with a thrombogenic platinum coil attached to a stainless steel delivery guide wire or mandrel. Once the coil is correctly positioned within the aneurysm, an electric current is applied to the guide wire, thereby electrolytically dissolving the soldered connection between the coil and delivery mandrel. The guide wire is subsequently removed and another coil can be advanced into the aneurysm until the aneurysm is completely excluded from the arterial circulation (i.e., no longer fills with contrast). A wide range of aneurysm morphologies can be treated because of the intrinsic compliance of the coil helix.
Treatment with GDC offers durable, long-term protection from aneurysm rebleeding. The rate of rebleeding following coil embolization is directly proportional to the percentage of the aneurysm that remains patent. Not surprisingly, the percentage of residual aneurysm patency following coil embolization is dependent on aneurysm neck size—the size of the opening connecting the aneurysm to the flowing blood. Small aneurysms with wide necks and large aneurysms (which typically have large necks as well) are most likely to be incompletely treated following coil embolization. To aid in the endovascular coil embolization of wide-necked aneurysms, balloon remodeling was introduced.
The balloon remodeling technique involves temporary inflation of a nondetachable balloon within the parent artery over the aneurysm neck during coil placement. The inflated balloon provides a resistive barrier to keep the coil within the aneurysm during and after deployment, preventing migration into the parent artery. The balloon
remodeling technique is particularly useful in treating ruptured aneurysms with challenging neck anatomy because of the necessity of double antiplatelet therapy (usually aspirin and clopidogrel) when a permanent intravascular stent is used. Disadvantages of the technique include potential ischemia secondary to blood flow arrest, migration of the coil mass after the balloon is deflated, and intimal damage and thrombosis related to repeated inflation of the balloon.
remodeling technique is particularly useful in treating ruptured aneurysms with challenging neck anatomy because of the necessity of double antiplatelet therapy (usually aspirin and clopidogrel) when a permanent intravascular stent is used. Disadvantages of the technique include potential ischemia secondary to blood flow arrest, migration of the coil mass after the balloon is deflated, and intimal damage and thrombosis related to repeated inflation of the balloon.
A more recent iteration in the evolution of endovascular aneurysm treatment involves moving the matrix of reconstruction from the aneurysm sac (endosaccular) to the parent artery lumen (endoluminal) via the use of a stent. The procedure involves placement of a permanent cylindrical cage or stent (current available devices include the Neuroform stent, Enterprise stent, LVIS Device, and LVIS Jr. Device) across the neck of an aneurysm to act as a resistive barrier, preventing the deployed coils from herniating into the parent artery. There are three traditional techniques for coiling with stent assistance. One technique involves placing a stent within the parent artery across the neck of an aneurysm and subsequently accessing the aneurysm with a microcatheter through the wall of the stent. An alternative approach involves using two catheters in which a coil catheter tip is advanced into the aneurysm after which a stent is deployed across the aneurysm neck, effectively “jailing” the first catheter between the stent and the arterial wall. The final technique involves coiling the aneurysm with balloon assistance and subsequently placing a stent across the neck of the aneurysm. Stent-assisted coiling offers several advantages over coiling alone or balloon remodeling including creating a permanent buttress for the coil mass, decreasing blood flow across the neck of the aneurysm (potentially facilitating hemostasis within the aneurysm), and providing a scaffold for endothelialization.
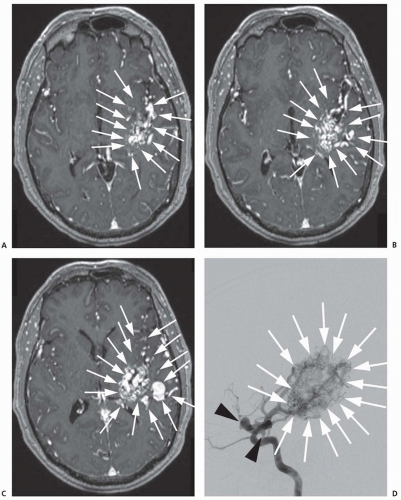
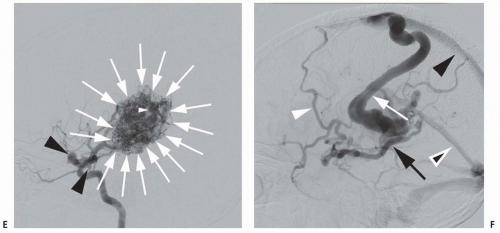
The newest technology in aneurysm treatment namely flow-diverting stents are designed as endoluminal-only aneurysm treatment constructs. The first of these devices, the Pipeline embolization device (PED), represents a major paradigm shift in the endovascular treatment of intracranial aneurysms (Fig. 24.3). The PED is a braided stent with approximately 30% total metallic coverage versus less than 10% for the Neuroform and Enterprise stents. The PED is ideal for the treatment of large or giant, wide-necked, and fusiform aneurysms, as the stent construct can be deployed across the diseased segment of artery, rapidly resulting in hemostasis and slowly endothelializing over the course of months. In a cohort of anterior circulation aneurysms with a mean aneurysm size of 18 mm and a mean neck size of 9 mm treated with the PED, 74% of aneurysms were completely cured at 6 months and 87% at 1 year, with a 6% incidence of major ipsilateral stroke or neurologic death. Although flow diversion is a promising technique, careful analysis and follow-up of treated patients is required to determine its safety and efficacy. Stent placement requires the long-term use of dual antiplatelet agents to prevent thrombosis, potentially problematic in the setting of acute SAH.
Choosing between endovascular and open surgical treatment of intracranial aneurysms is a complex decision, often dependent on local preferences and physician skill and experience. Nevertheless, two randomized trials have compared the two options in the setting of SAH. The International Subarachnoid Aneurysm Trial (ISAT) was a prospective, randomized, multicenter trial designed to compare the safety and efficacy of endovascular coiling with microsurgical clipping [Level 1].1,2 The study enrolled 2,143 patients between 1994 and 2003 with ruptured cerebral aneurysms and randomly assigned them to microsurgical clipping (n = 1,070) or endovascular coiling (n = 1,073). The primary outcome was death or dependence for activities of daily living at 1 year. This study showed that in this population of patients with small to moderate size anterior circulation aneurysms, endovascular coiling was safer
than surgical clipping: 24% of patients in the coiling arm were dead or dependent versus 31% in the clipping arm, a 23% relative risk reduction. At 5 years, there was a small but statistically significant increased risk of recurrent bleeding in the coil arm, but the risk of death was slightly albeit statistically significantly lower in the coiled group than in the clipped patients [Level 1].3 The Barrow Ruptured Aneurysm Trial (BRAT) reported remarkably similar results. BRAT prospectively treated 471 aneurysm patients, alternating between coiling and clipping. Overall, 34% of patients in the clipping arm were dead or dependent at 1 year compared to 23% in the coil arm, a statistically significant difference [Level 1].4 Nevertheless, 33% of those originally assigned to coiling crossed over to the clipping arm, reinforcing the need for open surgical treatment of difficult-to-coil aneurysms for the foreseeable future.
than surgical clipping: 24% of patients in the coiling arm were dead or dependent versus 31% in the clipping arm, a 23% relative risk reduction. At 5 years, there was a small but statistically significant increased risk of recurrent bleeding in the coil arm, but the risk of death was slightly albeit statistically significantly lower in the coiled group than in the clipped patients [Level 1].3 The Barrow Ruptured Aneurysm Trial (BRAT) reported remarkably similar results. BRAT prospectively treated 471 aneurysm patients, alternating between coiling and clipping. Overall, 34% of patients in the clipping arm were dead or dependent at 1 year compared to 23% in the coil arm, a statistically significant difference [Level 1].4 Nevertheless, 33% of those originally assigned to coiling crossed over to the clipping arm, reinforcing the need for open surgical treatment of difficult-to-coil aneurysms for the foreseeable future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree