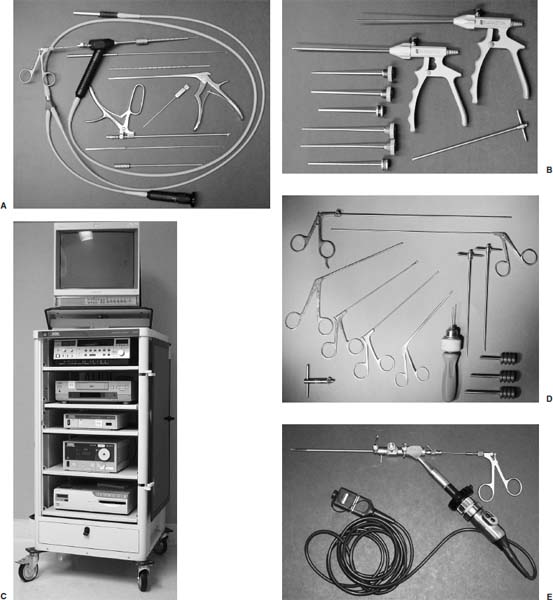
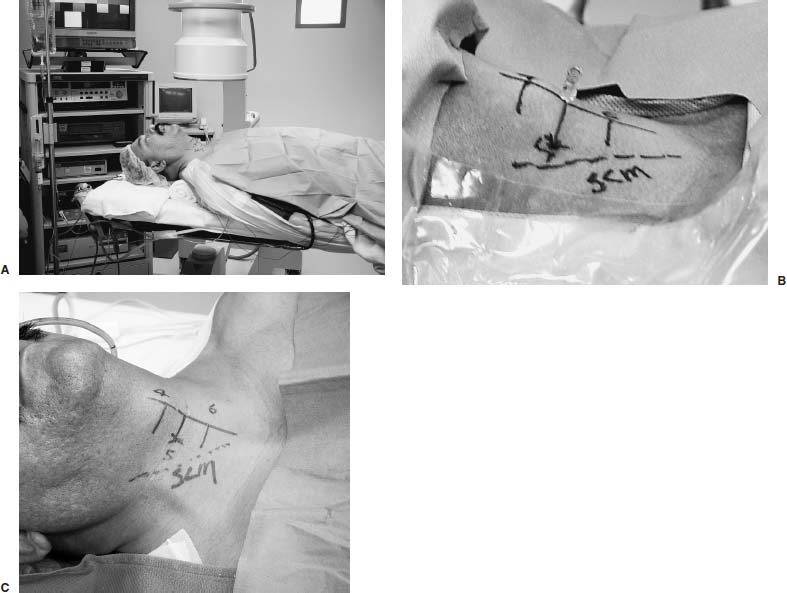
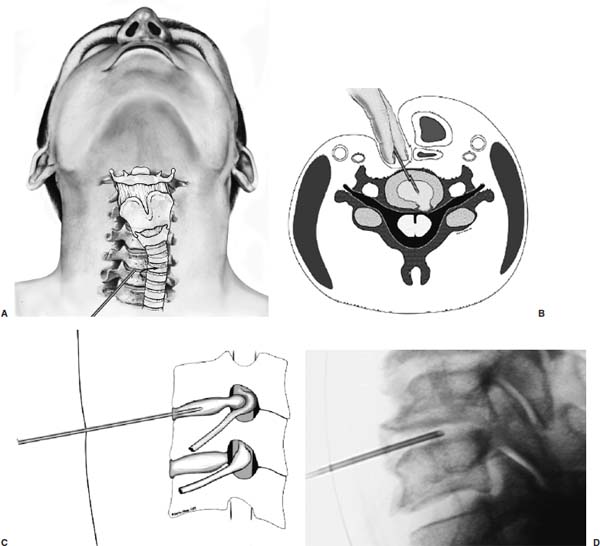
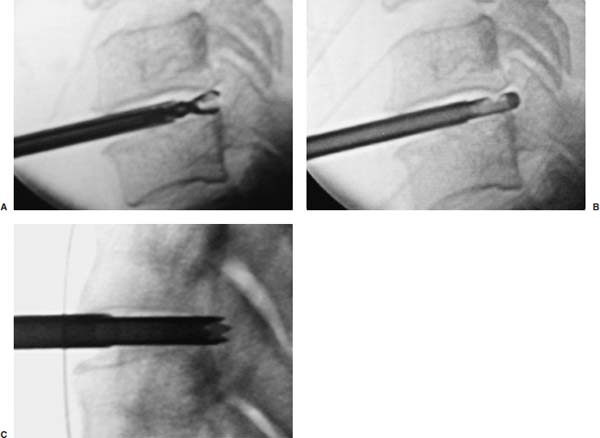
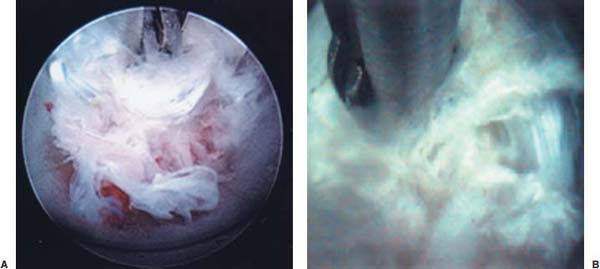
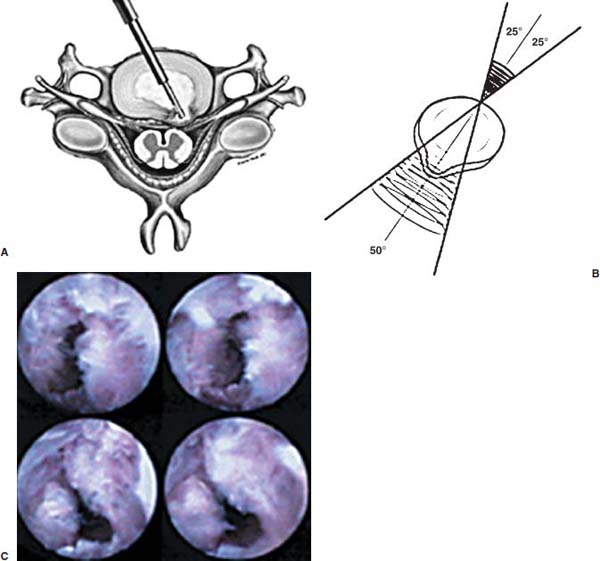
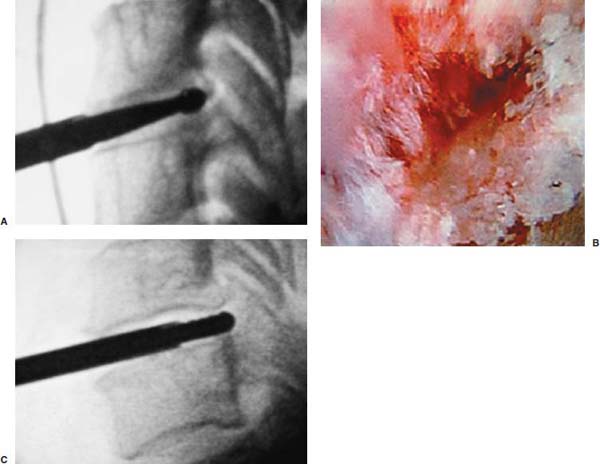
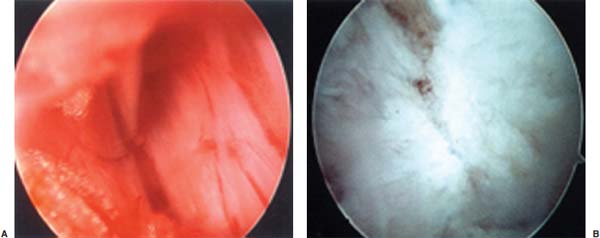
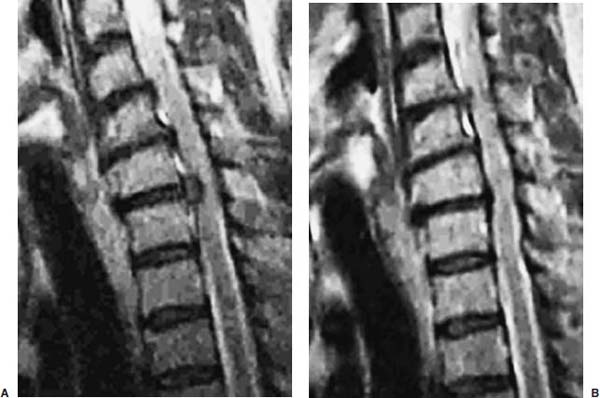
Anterior cervical diskectomy with or without bony fusion has been the standard treatment for cervical disk protrusions since the 1950s1–4; however, these operations are associated with significant local morbidity5,6 (e.g., graft collapse, graft extrusion, hardware failure, nonfusion with resultant instability, infections, esophageal perforation with infection, and permanent pain, peripheral nerve injury, or infection at the graft donor site). In contrast, anterior endoscopic cervical microdiskectomy (AECM)7,8 is a minimally invasive, outpatient procedure with much less morbidity, no graft donor site to cause secondary problems, and a significant decrease in the period of convalescence and costs. The evolution of spinal surgery is trending toward less-invasive techniques, with clever procedures not thought possible a few years ago.9–14 Advancements in microinstrumentation, fiberoptics, improved fluoroscopic imaging, and high-resolution digital video imaging endoscopy, along with the accumulation of experience in percutaneous lumbar diskectomy15–18 and spinal laser applications,18–20 have facilitated the development of AECM.7 In 1995, the use of a Holmium laser at low, nonablative levels of energy to cause contraction of the disk tissue, further reducing the size of the protrusion and hardening the disk (laser thermodiskoplasty) to prevent recurrence, was added to our standard protocol for percutaneous AECM. Anterior cervical fusion (ACF) is associated with a 15% or greater chance of junctional disk herniation at interspaces adjacent to fused levels, presumably because fusion places additional stress on adjacent vertebral segments. Minimally invasive endoscopic diskectomy does not affect the stability of adjacent vertebral segments. Multiple levels of symptomatic cervical disk disease do occur. ACF is often an unattractive treatment in the presence of multiple symptomatic disk levels in a patient, but AECM, a minimally invasive outpatient spinal surgery, can be safely utilized for these cases. This chapter will describe the surgical technique of AECM step by step, using current endoscopic equipment, instrumentation, surgical tools, potential surgical complications, and complication avoidance in the treatment of symptomatic herniated cervical disks. Indications The AECM approach is indicated in the following clinical situations7: FIGURE 5–1 Video digital endoscopy tower and anterior cervical endoscopic instruments. (A) Cervical 0-degree endoscopes and endoscopic instruments. (B) Diskectomes, working channel sets. (D) Diskectomy set, forceps, trephines, and burr. (E) Trichip digital camera with cervical 6-degree endoscope and forceps. (C) Video digital endoscopic tower. Surgical Equipment and Instruments The following surgical equipment and instruments are necessary to perform AECM: FIGURE 5–2 Holmium: yttrium-aluminum-garnet (YAG) laser equipment for laser thermodiskoplasty. (A) Holmium:YAG laser generator. (B) Right-angle (side-firing) laser probe. Anesthesia This procedure is done under local anesthesia with monitored conscious sedation or occasionally, if indicated, general anesthesia. Two grams of Ancef (cefazolin) and 8.0 mg of Decadron (dexamethasone) are routinely given intravenously at the start of anesthesia. The esophagus is made more palpable by inserting an esophageal or nasogastric tube. The pulse of the carotid artery may be augmented with use of sympathomimetics (e.g., ephedrine) to raise systolic blood pressure to a minimum of 130 mm Hg. Surface electroencephalogram (EEG) (SNAP, Nicolet Biomedical, Madison, WI) provides added precision for anesthesia. FIGURE 5–3 Patient positioning and localization. (A) Patient positioning. (B) Localization: skin marking. (C) Placement of needle. Patient Positioning The patient is placed in a supine position, with the neck extended over a rolled towel under the shoulders, on an adjustable radiolucent surgical table.7 A soft strap is placed over the forehead to stabilize it. The shoulders are distracted gently downward with tape (Fig. 5–3A). Digital C-arm fluoroscopy is used in anteroposterior (AP) and lateral planes to control the placement of all instruments throughout the operation. Electromysgraphy (EMG) needles are placed in areas of distribution of the nerve roots from the spinal levels being operated on to provide continuous neurophysiologic-neuromuscular monitoring throughout the procedure for added safety. Localization Initial localizing AP fluoroscopic x-rays are made with Allis clamps attached to the drapes and crossing the neck horizontally at the expected level. The midline, the levels, and the point of entry (operating portal) for surgery are marked on the skin with a marking pen (Fig. 5–3B,C). On the other hand, using sterile technique, the level of the disk can be accurately identified by inserting an 18-gauge needle into a disk under fluoroscopy (Fig. 5–4A–D), as described under Surgical Techniques. Surgical Techniques The initial point of entry (operating portal) is adjacent to the medial border of the right sternocleidomastoid muscle, where firm pressure is applied digitally in the space between that muscle and the trachea, pointing toward the vertebral surface (see Fig. 5–4A,B). The larynx and trachea are displaced medially and the carotid artery laterally (see Fig. 5–4A,B). The anterior cervical spine is palpated with the fingertips. An 18-gauge spinal stylette is passed transdermally into the disk space, and its position is confirmed fluoroscopically (see Fig. 5–4C,D). If a pain provocation test and diskogram have not been done preoperatively, they are done now. If results are confirmatory, the operation is performed. A 3 mm skin incision is made, and a narrow guidewire stylette is passed through the needle, which is then removed. A 2.5 mm or 3.5 mm internal diameter cannula/dilator is introduced over the stylette and into the disk interspace. FIGURE 5–4 Surgical technique for anterior medial approach for needle and stylette placement into the disk. (A) Retraction of trachea/esophagus for needle placement—frontal view. (B) Retraction of trachea/esophagus for needle placement—axial view. (C) Placement of the stylette into disk space—lateral view. (D) Placement of the stylette—fluoroscopic lateral view. A standard number 18 guidewire replaces the narrow one. A trephine replaces the dilator and incises the anulus. Under fluoroscopic and endoscopic visualization, minicurettes and forceps (Figs. 5–5A, 5–6) loosen and remove disk fragments prior to introducing the diskectome through the cannula to remove the disk. The diskectome employs a high-pressure irrigation-suction system and a guillotine-cutting blade (Figs. 5–5B, 5–7A). The instruments removing the disk material are moved in a “critical fan sweep maneuver” with a 25-degree “rocking” excursion of the cannula hub from side to side to increase the area of total disk removed up to approximately a 50-degree inverted cone-shaped area within the disk space (Fig. 5–7B).7 All maneuvers are closely monitored with fluoroscopy and by endoscopy. Trephines with more aggressive teeth are used to remove bone spurs or osteophytes. Very large bone spurs can be removed with a short cannula system with a burr (Fig. 5–8). The holmium: yttrium-aluminum-garnet (YAG) laser with right-angle (side-firing) probe facilitates removal of disk material further. In addition, it is used at nonablative levels of laser energy (Table 5–1) for laser thermodiskoplasty [i.e., collagen and disk shrinking (Fig. 5–7C) and tightening effect to further decompress and to harden the disk]. Endoscopy is used for direct visualization (Fig. 5–9A,B) and for confirmation of diskectomy and laser thermodiskoplasty. Again, after using the diskectome for laser disk debris removal in the disk space, the probe and cannula are removed. Marcaine (0.25%) is infiltrated subcutaneously around the wound. The tiny incisional wound is closed with a bandage. FIGURE 5–5 AECM with forceps, diskectome, and trephine under fluoroscopy. (A) Microdiskectomy forceps. (B) Diskectome. (C) Trephine. FIGURE 5–6 (A,B) Endoscopic views of anterior endoscopic cervical microdiskectomy with forceps removing disk material. FIGURE 5–7 Surgical technique of laser thermodiskoplasty, fan sweep maneuver, and endoscopic views of disk shrinkage. (A) Sidefire laser probe in action. (B) “Fan sweep maneuver” of instrument increased disk removal and shrinkage. (C) Laser used to shrink and tighten the disk besides “purse string” of the disk defect. Postoperative Care Patients are ambulatory within 1 hour postoperatively and discharged subsequently. They may shower the following day. A soft cervical collar is used for 2 to 3 days, or as needed. An ice pack is helpful. Mild analgesics and muscle relaxants are required at times. Progressive neck exercise begins on the second postoperative day. Patients are allowed to return to work in 1 to 2 weeks, provided heavy labor and prolonged sitting are not involved. This procedure can be extremely gratifying for patients. FIGURE 5–8 Rasp and burr used to remove osteophyte. (A) Burr to remove osteophyte. (B) Endoscopic view of disk space after osteophyte removal. (C) Rasp in action. Ninety-four percent of surgical patients with single disk problems have good-to-excellent relief of symptoms postoperatively,7,19 resuming usual activity in a few days and full active lives in 3 to 7 weeks. Complications and Avoidance A thorough knowledge of the AECM procedure and surgical anatomy of the neck, careful selection of patients, and preoperative surgical planning with appropriate diagnostic evaluations facilitate the AECM and prevent potential complications. All potential complications of open anterior disk surgery are possible, but they are rare or much less frequent in AECM. Infection Infection can be avoided by careful sterile technique, using prophylactic antibiotics IV intraoperatively and the much smaller incisional area. Infection and complications secondary to a donor graft site are obviated. Aseptic diskitis can be prevented by aiming the laser beam in a “bowtie” fashion to avoid damaging the end plates (at 6 and 12 o’clock). FIGURE 5–9 Endoscopic views of uncinate joint and nerve root after decompression, and fissure in cervical disk. (A) Uncinate joint and nerve root after endoscopic microdecompression. (B) Intraoperative endoscopic view of fissure in cervical disk. Hematoma (Subcutaneous and Deep) Hematoma occurs post-ACF and may occur with minimally invasive spinal surgery, but is minimized by careful technique, by not prescribing aspirin or nonsteroidal antiinflammatory drugs (NSAIDs) within 1 week prior to surgery, by application of gentle digital pressure, or by placing an IV bag over the operative site for the first 5 minutes after surgery and application of an ice bag thereafter. Vascular Injuries Vascular injuries are extremely rare when care is taken to locate and protect the carotid artery, jugular vein, and other vascular structures, including the vertebral artery in the foramen transversarium laterally and the inferior and superior thyroid arteries. No carotid artery injury has been reported in the United States. The carotid sheath should be identified and protected under the surgeon’s fingers. If carotid arterial pulsation is hard to palpate, it can be augmented by IV ephedrine. No prolonged retraction of the carotid sheath and artery is required because it is in anterior cervical fusion; hence, direct trauma and embolic complications involving carotid vessels are unlikely. Neural Injuries Neural injuries are extremely rare with minimally invasive approaches. No spinal cord injuries have been reported. Nerve root and spinal cord injury, although possible, can be avoided with continuous intraoperative neurophysiologic monitoring [EMG/nerve conduction velocity (NCV)] and direct endoscopic visualization. Neural complications of ACF, including hypoglossal, spinal accessory, and phrenic, auricular, and cutaneous nerves, can be prevented by careful technique. Recurrent laryngeal nerve injury, although a recognized complication of ACF, is extremely rare with AECM. One case with hiccough and one with hoarseness have postoperatively occurred transiently out of 1200 cases of AECM. Sympathetic Nerve Injuries Sympathetic nerve injuries are extremely rare, but they can occur from injury to cervical sympathetic and stellate ganglia. One incidental transient Horner’s syndrome or oculosympathetic dysfunction lasting 1 day following AECM was noted. Excessive Sedation Excessive sedation can be avoided by surface EEG monitoring, providing more precise estimation of the depth of anesthesia, reducing the amount of anesthetics, and preventing excessive or insufficient sedation. Operation on the Wrong Level A major complication of ACF or AECM is operating at the wrong level and with improper instrumentation. Proper utilization of digital C-arm fluoroscopy for anatomical localization avoids complications caused by poor placement of instruments or operating at the wrong disk level. Dural Tears Dural tears have not been reported in AECM. Soft Tissue Injuries Although soft tissue injuries may occur due to prolonged forceful retraction, as occurs in ACF operations, they are not an issue with AECM. Failure of fusion, collapse of the bone plug, migration of the bone plug, and hardware failure similarly cannot occur. Dysphasia and Postoperative Airway Obstructions Due to Edema Airway obstructions may occur, but they can be avoided by careful surgical procedures and identification of these organs. Esophageal and tracheal injury due to edema or perforation can be avoided by careful palpation and digital retraction at the site of needle insertion. Having the anesthesiologist place a nasogastric tube into the esophagus aids in identifying and retracting that structure by palpation. Inadequate Decompression of Disk Material Inadequate decompression of disk material is minimized by using multiple modalities and instruments (e.g., forceps, trephining the posterior ligament, diskectome, burr and rasp, and laser application to both vaporize tissue and perform thermodiskoplasty). Thyroid Gland Injury Injury to the thyroid can be avoided by approaching the glands posteriorly and not encountering the parenchyma. A large goiter requires special care because of its vascularity. FIGURE 5–10 Large 8 mm herniated C6–C7 disk compressing spinal cord. (A) Pre- and (B) postoperative magnetic resonance imaging scans. Advantages of AECM The advantages of AECM are those of minimally invasive spinal surgery7: A 62-year-old female patient complained of progressive neck, shoulder, and bilateral arm pain for 3 years, with increasing stiffness of the legs, along with stool and urinary incontinence for 3 months. The left biceps reflex was reduced, and knee and ankle reflexes were 2+. Hypoalgesia was present in the left middle and ring fingers. Babinski’s reflex was equivocal on the right. Mild spastic gait was evident. EMG was abnormal in a left C6–C7 distribution. MRI (Fig. 5–10A) confirmed a large 8 mm herniated C6–C7 disk causing spinal cord compression, a 2 mm disk protrusion at C4–C5, and a 2 to 3 mm protrusion at C5–C6. Provocative diskogram and anterior endoscopic microdecompressive diskectomy with laser thermodiskoplasty were performed. Very brief transient worsening of gait was followed postoperatively by rapid improvement of all symptoms and disappearance of incontinence by the third postoperative day. She was asymptomatic and free of pain at follow-up 1 year later. She had resumed all her normal activities. Comparative pre- and postoperative MRI scans showed complete removal of the large herniated C6–C7 disk (Fig. 5–10B). Conclusion Anterior endoscopic cervical microdiskectomy, which represents a new procedure for treating symptomatic herniated cervical disks anteriorly through an endoscope, aims to reduce tissue trauma from conventional open cervical surgery. Low-energy nonablative laser is applied for shrinkage and tightening of the disk (laser thermodiskoplasty). With appropriate endoscopic spinal surgical training, thorough knowledge of the AECM procedure, and surgical experience, AECM is a safe and efficacious procedure. This minimally invasive and less traumatic outpatient procedure results in less morbidity, more rapid recovery, and significant economic savings. REFERENCES 4. Robertson JT. Anterior removal of cervical disc without fusion. Clin Neurosurg. 1973;20:259–261. 10. Davis GW, Onik, Helms O. Automated percutaneous discectomy. Spine. 1991;16:359–363. 12. Ascher PW. Application of the laser in neurosurgery. Laser Surg Med. 1986;2:91–97.
5

Anterior Endoscopic Cervical Microdiskectomy


< div class='tao-gold-member'>
Anterior Endoscopic Cervical Microdiskectomy
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree