CHAPTER 302 Anterior Lumbar Instrumentation
Indications
Neoplasm
The spinal column is the most frequent site of bony metastases1; up to two thirds of these arise from breast, prostate, lung, and hematopoietic cancers.1–6 Primary tumors of the spine, on the other hand, are extremely rare. Less than 10% of reported bone tumors and soft tissue sarcomas involve the spine.7,8 Clinical manifestations of spinal tumors result from expansion of the periosteum or cortex, pathologic compression fractures, spinal instability, spinal deformity, and direct compression of neural elements. By far the most common symptom produced by either primary or metastatic lesions is pain,9–12 stereotypically a constant pain that is not relieved by rest and intensifies at night. Progression of the tumor beyond the confines of the vertebral body may produce myeloradiculopathy because of direct compression of neural elements or compromise of vascular supply. Acute partial loss of neurological function may be an indication for urgent surgical intervention because studies have shown improvement in neurological function following early surgical treatment in such circumstances.13,14
Numerous series have reported the successful treatment of spinal neoplastic disease with a ventral or ventrolateral approach.6,14–22 Prognostic factors, including tumor type and location, preoperative neurological status, overall life expectancy and medical condition correlate with surgical outcome23,24 and should be carefully considered in formulating a management strategy for each patient.
Trauma
Compared with fractures of the thoracolumbar junction, fractures of the lumbar spine distal to L2 are relatively uncommon, with less than 4% of all fractures involving L3-5.25,26 Certain mechanisms of high-energy traumatic injury tend to be associated with both thoracolumbar and lumbar fractures. Falls from a significant height that produce a substantial axial load may result in unstable burst fractures. Passengers wearing lower abdominal seat belts during motor vehicle accidents may sustain flexion or flexion-distraction injuries to the lumbar or thoracolumbar spine, with the seat belt acting as a fulcrum.
The decision to treat a lumbar spine trauma patient operatively is based on multiple factors. In addition to the presence of a neurological deficit, the extent of spinal canal encroachment or compromise, loss of vertebral body height, degree of local kyphotic deformity, and fracture pattern may guide treatment decision making.27–38 Various classification schemes have been proposed for spinal fractures, many of which are based on Denis’ three-column model of spinal stability and on the mechanism of spinal injury.39–43 Generally, a fracture that compromises the anterior and middle columns is likely to be unstable, but isolated anterior column involvement causing significant kyphosis may also warrant surgical intervention to prevent the development of a progressive deformity.44 The surgeon must also decide whether a stand-alone anterior construct is appropriate or if a circumferential (anterior-posterior) construct and arthrodesis are necessary.
Surgical decompression is the first-line treatment for a patient with an acute partial neurological deficit due to neural compression following trauma. Emergent decompression is recommended for a progressive neurological deficit and a grossly unstable spine. The indications for operative treatment of patients with fixed neurological deficits are less clear. Advocates who favor delaying surgery believe that there is a reduced risk of neural injury and intraoperative blood loss with surgery after the acute period.45,46 Proponents of early surgery, on the other hand, contend that it provides the best opportunity for neurological recovery and that delaying intervention may make it more difficult to achieve fracture reduction or spinal correction. Patients may also be mobilized early after surgery, thereby avoiding the complications of prolonged bed rest.47
Degenerative Disease
Degenerative disease is a common condition of the lumbar spine, which is often characterized by chronic, progressive instability.48 Reconstruction of the anterior column can effectively improve sagittal balance, restore lumbar lordosis, and enlarge the neural foramina, reversing the pathologic consequences of degenerative processes.49,50 Anterior interbody fusion may be indicated for a wide range of degenerative pathology including degenerative disk disease, lumbar instability, iatrogenic instability, pseudoarthrosis following posterior arthrodesis, or a grade I or II spondylolisthesis.50–58 There are, however, several relative contraindications to anterior interbody fusion procedures including severe osteoporosis, grades III and IV spondylolisthesis, and extensive vertebral body destruction. In cases of severe degenerative destruction of vertebral bodies, or a significant local spinal deformity, multisegmental ventral instrumentation constructs may be necessary, either alone or in conjunction with posterior fixation.
Infection
Bacterial organisms, usually Staphylococcus aureus, are the most common causes of spinal infections, and most spinal infections are treated with intravenous antibiotics and immobilization before the onset of a neurological deficit or spinal deformity.59–61 Operative indications include a progressive neurological deficit, a significant spinal deformity, intractable pain, or, more rarely, progression of the infection despite appropriate antibiotic therapy. Because infections of the vertebral body affect the anterior and middle columns, the surgical approach must provide access to the ventral spine to allow complete débridement of the active infection. This often involves discectomies and a corpectomy of the involved vertebral body followed by a fusion. Evidence suggests that the rate of postoperative infection is not increased in the presence of metallic implants provided débridement has been adequately performed.59,62
Surgical Approaches
The decision of which approach to use is influenced primarily by which lumbar levels are involved and by the goals of surgery. Over the years, several ventral approaches for access to the anterior lumbar spine have been developed and refined.58,63,64 The approach selected should provide maximal visualization of the lesion and regional anatomy. For instance, exposure of the thoracolumbar junction for access to L1 requires a thoracoabdominal approach and release of the diaphragm. Lesions located between L2 and L5 can be addressed through a flank or paramedian incision and a retroperitoneal approach. If midline exposure of L2 to L5 is required, a transperitoneal approach is often most direct. The side of the approach is ultimately dictated by the location and nature of the pathology, but a left-sided approach is usually preferred because the liver is avoided and the aorta is easier to mobilize and less susceptible to injury than the vena cava. Our practice is to use a general or vascular surgeon for anterior approaches to the lumbar spine to ensure a safe and adequate exposure. We scrub in, however, as assistants, to gain experience with the approaches and to help tailor the exposure to the requirements of the individual case.
Thoracoabdominal Approach (T11-L2)
The thoracoabdominal approach provides for the best exposure of T12-L2. This approach requires a rib resection; generally, resection of the rib two levels above the level of primary pathology is performed.65–67 Hence, resection of the ninth rib provides the best window of access to T11-12, and is accomplished through a transthoracic approach, whereas exposure of T12-L1 may be accomplished via a thoracoabdominal 10th rib approach. Generally, thoracoabdominal exposure can be extended distally to gain access to additional lumbar levels without significant difficulty.
The patient is placed in the lateral decubitus position. A left-sided approach is usually preferred to avoid the inferior vena cava and the liver. An incision is marked using fluoroscopy and is made from the lateral border of the paraspinal musculature along the 10th rib to the junction of the rib and the costal cartilage.68 A circumferential subperiosteal rib dissection is performed using monopolar cautery and Doyen dissectors with care taken to avoid the neurovascular bundle that lies on the inferior aspect of the rib. The rib is divided posteriorly at the angle of the rib and at the junction of the rib and costal cartilage. The rib is removed, the remaining costal cartilage is divided lengthwise with a knife or scissors, and the flaps of cartilage are retracted. The retroperitoneal space is identified with blunt dissection deep to the costal cartilage and the peritoneum is dissected off the inferior surface of the diaphragm. The peritoneum is then retracted medially, and the abdominal musculature is divided in layers, revealing the diaphragm. The endothoracic fascia within the rib bed is divided along with the parietal pleura, which is deep to it. The soft tissue is swept off the thoracic and abdominal surfaces of the diaphragm, which is then incised circumferentially leaving a cuff of muscle of approximately attached to the chest wall. The crus of the diaphragm is cut and elevated off the spinal column. Alternatively, the diaphragm is bluntly stripped from the chest wall with a Cobb elevator or sponge stick. This technique obviates the necessity of reapproximating the diaphragm at closure. A large Deaver retractor is used to retract the peritoneal sac anteromedially, and a large rib retractor opens the 10th rib space to reveal the thoracolumbar junction. In the lumbar spine, the psoas must be elevated dorsolaterally off the vertebral bodies to allow full visualization as far distally as the lesion dictates, taking care not to injure the lumbar plexus that runs within the muscle or the genitofemoral nerve that runs on its surface. A table-mounted retractor with multiple adjustable arms is used to maintain exposure during the procedure.
Retroperitoneal Flank Approach (L2-4)
The patient is placed in a lateral decubitus position with appropriate padding to prevent pressure ulcerations and neuropathies. If an electric operating table is used, the patient is positioned so that flexion of the operating table opens the space between the iliac crest and costal margin; otherwise a small rolled towel or pad may be used to achieve the same position. The incision begins in the midaxillary line between the inferior margin of the ribs and the iliac crest and follows an inferior oblique course to the lateral edge of the rectus sheath. The level of the incision is determined using fluoroscopy based on the desired level of exposure (Fig. 302-1). For lesions of the upper lumbar spine, the incision should be made above the umbilicus along the 11th or 12th rib. Occasionally a portion of the rib may be osteotomized or excised to improve exposure and can then be subsequently used as graft material. For lesions in the midlumbar spine, the incision starts at the level of the umbilicus. The lower lumbar spine is accessed through an incision superior to the midpoint between the umbilicus and the symphysis pubis. If exposure distal to L4 is required, the medial aspect of the incision can be extended caudally, essentially adding a paramedian approach type of incision to the traditional flank incision.
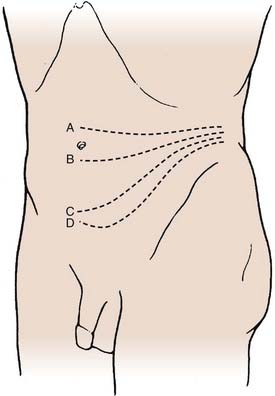
FIGURE 302-1 Transverse incisions for the retroperitoneal approach: L2-3 (A), L3-4 (B), L4-5 (C), and L5-S1 (D).
(From Benzel EC. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone; 1999.)
The underlying musculature, including the latissimus dorsi, serratus posterior inferior, external oblique, and internal oblique muscles, are transected in line with the skin incision. The transversalis fascia is identified and divided to enter the retroperitoneal space. The underlying peritoneum is identified as a semitranslucent layer and carefully separated from the abdominal wall using blunt dissection with a finger or a sponge stick. Adhesions, particularly toward the midline at the rectus sheath, may require sharp dissection. A plane between the quadratus lumborum muscle and retroperitoneal organs is developed, and the viscera, including the kidney, perirenal fat, and ureter, are retracted medially (Fig. 302-2). Dissection dorsal to the psoas muscle is avoided because this plane ends in a blind pouch. The dissection is continued caudally, mobilizing the peritoneum off the posterior abdominal wall to the level of the sacrum. The retroperitoneal organs and peritoneal contents are padded with moist sponges and retracted medially and positioned outside the operative field using a table-mounted retractor system. The dissection continues dorsomedially to identify the psoas muscle and genitofemoral nerve. This nerve lies along the ventral surface of the psoas muscle and should not be injured (Fig. 302-3). The psoas muscle may require dorsolateral mobilization with a Cobb elevator and monopolar coagulation to expose the spine. Overly aggressive retraction of the psoas should be avoided to prevent injury to the lumbosacral plexus located within the muscle. The sympathetic trunk, which lies just medial to the psoas along the ventrolateral surface of the vertebral bodies, should be preserved if possible (see Fig. 302-3). Division of the sympathetic trunk may result in unilateral decreased sympathetic tone in the lower extremity. Patients may complain of a cold contralateral leg because the retained sympathetic activity in the normal limb maintains vasoconstriction. At the lower lumbar regions, particularly L4-5, the common iliac vessels lie along the lateral aspect of the vertebral bodies and may require medial or lateral mobilization to increase exposure.
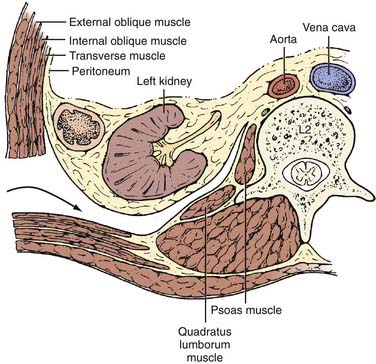
FIGURE 302-2 Axial view of ventrolateral retroperitoneal approach.
(From Benzel EC. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone; 1999.)
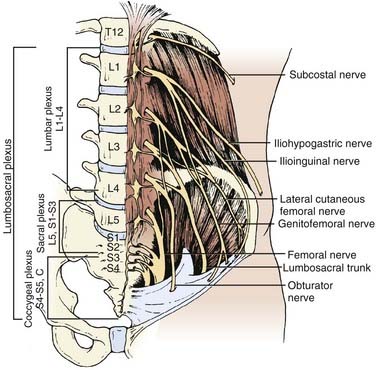
FIGURE 302-3 The lumbosacral plexus.
(From Benzel EC. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone; 1999.)
Transpsoas Approach (L1-5)
In recent years the field of minimally invasive surgery has expanded to involve surgical approaches to the spine as surgeons and patients seek novel methods to reduce tissue trauma during surgery, lessen postoperative discomfort, and shorten hospital stays. To this end, a novel approach through the psoas muscle offers a lateral approach that may be accomplished through one or two small (3- to 4-cm) incisions using tubular retractors and avoids the need for either a transperitoneal or retroperitoneal anterior approach.69
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








