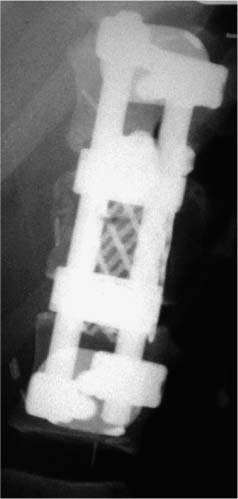
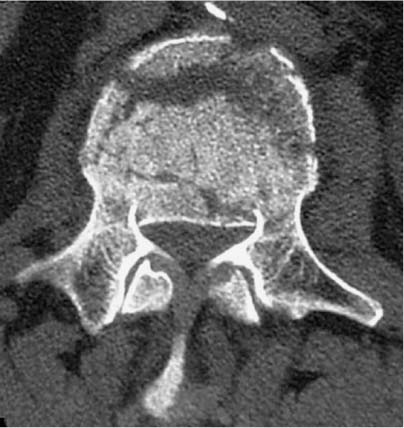
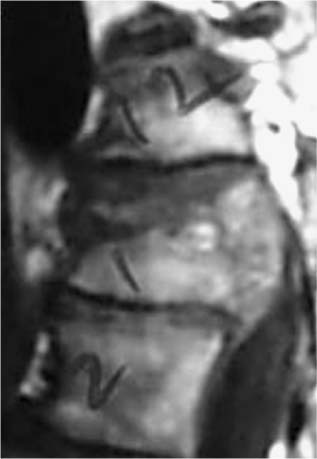
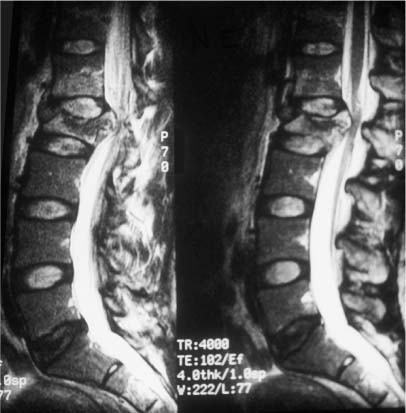
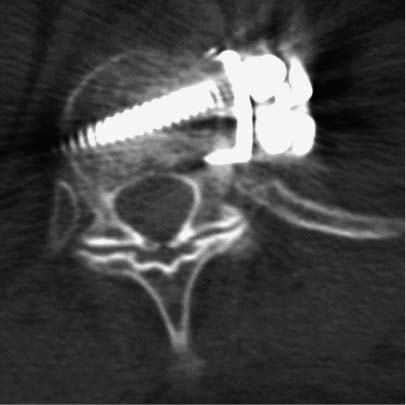
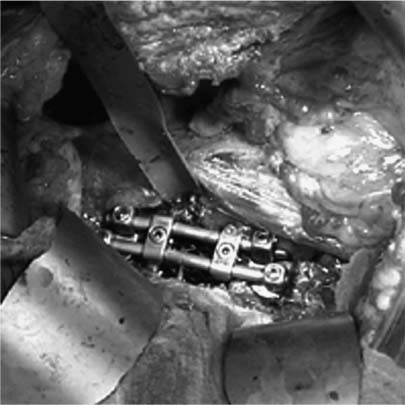
37 Royle1 was the first to describe an anterior spinal cord decompression by removal of an accessory vertebra in 1928. Several years later the technique was used to decompress a spinal tuberculous abscess.2 As anterior approaches to the thoracolumbar spine have become more popular, instrumentation systems were developed to ensure stability after decompression and grafting to obviate the need for prolonged bedrest and casting. Humphries et al3 in 1961 were the first to describe the use of anterior instrumentation in the lumbar spine. They were dissatisfied with the results of posterior fusions. Therefore, they considered attempting an anterior fusion, through a transperitoneal approach. They attached a plate via unicortical screws to the anterior vertebral bodies in the lumbar spine after discectomy. The plate design also allowed it to be adjusted into compression. Of 23 patients who underwent operation, 18 went on to definite bony union, whereas three remained questionable and two developed pseudarthrosis. No failure of instrumentation was noted. Further strides in instrumentation occurred when Dwyer et al4 and Zielke5 developed anterior screw fixation devices to aid in the correction of scoliosis; however, this instrumentation system was ineffective in cases of significant spinal instability. Rigid anterior fixation systems as developed by Slot in 1981, Kaneda in 1982, Dunn in 1984, Kostuik and Harrington in 1988, and Yuan (Syracuse I-plate) in 1987 contributed to the reconstruction after anterior procedures. Biomechanical studies have demonstrated the superiority of dual screw-rod systems over posterior instrumentation alone for stabilizing anterior and middle spinal column disruption.67 The surgeon today has many choices in anterior screw-rod systems. Current rod systems in use include the Slot-Zielke (Osteotech, Shrewsbury, NJ), Kaneda (AcroMed, Cleveland, OH), TSRH system (Sofamor-Danek, Memphis, TN), Kostuik-Harrington (Zimmer, Warsaw, IN), Bad Wildungen Metz-BWM (Howmedica, Kiel, Germany), anterior ISOLA system (AcroMed, Cleveland, OH), Synergy System (Cross Medical Products, Columbus, OH), and Antares (Sofamor-Danek, Memphis, TN). Anterior decompression and instrumentation are indicated in a variety of disease processes. Fracture with compression of the spinal cord, whether secondary to trauma or to other pathologic causes, is the most frequent indication. Infection, disc herniation, and deformity are also well-established indications. An anterior decompression is desirable when the pathologic process principally involves the vertebral body or includes the midline disc space, resulting in compromise of the ventral spinal cord and nerve roots. Sonntag and Hadley8 noted that traumatic injuries to the thoracolumbar junction (T10–L2) are the most common type of injury to this region treated by spine surgeons (Figs. 37-1 and 37-2). This region follows the cervical spine as the second most common region to be injured by trauma in adults, representing ~20% of cases.8 The thoracolumbar spine is a transition zone between the rigid kyphosis of the thoracic spine and the mobile lordosis of the lumbar spine and is at higher risk for injury. The thoracic spine has added protection from injury by the rib cage and its associated articulations, whereas the lower lumbar spine has relative protection from injury by its larger size of the vertebrae and its attachments to the iliac crests via the iliolumbar ligaments. Injuries to T12 are the most frequent and are associated with the highest incidence of neurologic impairment. FIGURE 37-1 An axial computed tomography (CT) scan demonstrates a burst fracture of the lumbar spine. As mentioned above, spinal cord compression may occur secondary to pathologic processes such as metastases. The spine is the most common site for skeletal metastases irrespective of the primary tumor. Approximately 75% of metastases are from breast, lung, lymphoma, myeloma, thyroid, kidney, and prostate.9 The vascular active marrow of the vertebral body serves as a perfect host to cancer cells. Metastatic disease, therefore, preferentially starts within the vertebral body as opposed to the posterior elements, which have been reported to be involved one seventh as frequently.9 Compression, therefore, occurs preferentially anterior to the spinal canal and decompression should involve the anterior column (Fig. 37-3). FIGURE 37-2 A sagittal CT reconstruction shows a burst fracture of the thoracolumbar spine with retropulsed bone fragments. Several authors have commented on their experiences with posterior decompression in the face of anterior disease with ventral spinal cord compression. Gilbert et al10 reported that radiotherapy alone was just as effective as laminectomy with or without radiation. They also found that more patients were able to regain functional ambulation postradiation than with laminectomy and radiation. Findlay11 reviewed the literature concerning management of malignant spinal cord compression. One third of patients with no vertebral body collapse who underwent laminectomy and radiotherapy became ambulatory from a nonambulatory status, whereas 25% developed neurologic decline. Patients with vertebral collapse treated similarly developed paraplegia in 54% of cases and became ambulatory in only 15% of cases. An increased incidence of instability was noted in patients with vertebral body collapse who were treated with laminectomy.11 An increased incidence of instability postlaminectomy would logically be anticipated in a process that, as Kakulas and associates12 noted, causes preferential anterior versus posterior vertebral body collapse and subsequent kyphosis at the segment. By performing laminectomies, the posterior elements have now been destabilized, causing two- or three-column disruption depending on the integrity of the middle column. Hall and Mackay13 evaluated the efficacy of laminectomy in relation to location of the metastatic tumor within the spinal canal and found greater than one third of patients with posterior or laterally placed tumors improved neurologically compared with only 9% of patients with anterior tumors. Many surgeons now perform anterior decompressions and stabilizations as a single-stage procedure. Moore and Uttley14 noted in their prospective review of metastatic disease treated with anterior decompression and stabilization that 62% of nonambulatory patients became ambulatory postoperatively and 71% with intractable pain became pain free. Other reviewers have had similar results.15,16 FIGURE 37-3 T2-weight sagittal magnetic resonance imaging (MRI) of bony collapse and destruction from metastatic disease. Indications for surgery of metastatic disease include intractable pain, impending fracture, compression of the spinal cord/cauda equina by bony elements, even when involved by a radiosensitive tumor, and relief of compression by a tumor that has failed to respond to radiotherapy or is known to be radioresistant. Patients with an anticipated life expectancy of less than 6 months may be considered for palliative treatment only. Ultimately the goal of surgery is to decompress the neural elements and stabilize the spinal column. Decompression of anterior pathology can be performed either via an anterior approach with the aid of a thoracic/vascular surgeon or posterior via a retroperitoneal costotransversectomy. Instrumentation of the spine for stabilization can occur via either route, although the application of an anterior screw-rod construct usually requires an anterolateral approach. The approach used depends on the individual patient and the comfort of the surgeon with that approach. Approach to the thoracolumbar junction may occur from either a right- or left-sided flank incision. In general, a left-sided approach is preferred because it obviates the need to retract the liver and involves dissection of the aorta rather than the vena cava. The aorta is thick walled and fairly easily mobilized, whereas the vena cava is thin walled and difficult to repair if it is injured. The patient is placed in the lateral decubitus position with the left side up. A beanbag can be used to support the body in this position and resists patient movement during surgical manipulation of the patient. Care must be taken to adequately pad the arms, feet, and knees, especially the peroneal nerve of the dependent lower extremity. An axillary roll is placed under the dependent right axilla, and an airplane may be used to support and pad the overlying left arm. The level of interest is placed over the break in the table, and the table flexed to help open up the spine in the area of interest. In most cases, an 11th rib exposure is used, saving the rib to be used as autograft. A thoracic or vascular surgeon may serve to provide excellent exposure for the spine surgeon. The spine surgeon must evaluate the adequacy of exposure to ensure that the proper trajectory will be obtained in placing instrumentation. If exposure is inadequate, improper trajectory of the screws may result. If exposure is limited, a separate stab incision at the rib space either superiorly or inferiorly can aid in instrument placement. Dissection proceeds by retracting the psoas muscle laterally and exposing the vertebral bodies. Ligation of the segmental vessels exposes the lateral aspect of the pedicle and the neural foramina. At the thoracic levels the rib head will need to be removed to expose these landmarks. Discectomies are now performed on either side of the vertebral body to be removed. Care must be taken to remove as much disc as possible from the end plates to ensure that bone removal is not hindered by tenacious disc material. In trauma cases and tumor cases where an intralesional excision is acceptable, a corpectomy is performed using a combination of rongeurs, Kerrison punches, and a high-speed drill. The corpectomy should proceed until there is a thin shell of bone overlying the posterior longitudinal ligament. Removal of the bone can then proceed at the upper lateral border until the underlying posterior longitudinal ligament or dura is encountered. Down-angled curettes may then be used to remove remaining bone away from the spinal canal into the resection cavity. Kerrison rongeurs can also be used to accomplish the decompression. Care must be excised to not accidentally catch the exiting nerve root in the rongeur. For cases of spondylectomy, see Marmor et al17 for a guide to the procedure. After the corpectomy has been performed, the posterior bolts for the screw-rod construct may be placed. These are positioned 8 to 10 mm inferior and anterior to the superior end plate and pedicle, respectively, for the superior vertebra, and 8 to 10 mm superior and anterior to the inferior end plate and pedicle, respectively, for the inferior vertebra. Some instrument systems come with a plate that is placed adjacent to the end plate (caudal and cephalad plates are made). Once the plate is tapped into position the posterior bolts are placed through preexisting holes. The bolts are angled 10 degrees away from the spinal canal. The angulation avoids possible injury to the posterior spinal canal. Bolt length can be calculated from preoperative CT scans. The bolt should engage the opposite cortex (Fig. 37-4). Intraoperative radiographs are obtained to ensure adequate distal cortex engagement without causing injury to vascular structures. If a question exists regarding screw or bolt prominence, direct palpation of the screw on the opposite side of the vertebral body cortex can be performed using gentle dissection. These bolts may then be used for distraction of the space, thus correcting any kyphosis, and aid in placement of an intervening graft. If polymethylmethacrylate (PMMA) is to be used, short-segment Steinmann pins can be placed into the end plates of the superior and inferior vertebra, creating a bridging construct and template for the PMMA to be placed on. If a bone graft or cage is to be placed, an appropriately sized piece is fashioned and tapped into place. Distraction can then be relaxed once the intervening graft is firmly in place and the table straightened out to help create compression across the graft. Care is taken to ensure that no underlying compression of the anterior dural sack occurs from the graft. The overlying rod may then be placed within the bolts and secured. Compression may be performed across an underlying bone or cage construct before final tightening of the overlying screw-rod construct is performed. PMMA does not require compression. The anterior screws are then placed perpendicular to the vertebra at the anterior aspect of the vertebral body and slightly inferior to the posterior screws. Again, these screws engage the opposite cortex, and intraoperative x-rays are taken to confirm adequate screw/bolt position. Once the screws are in a good location, an overlying rod is placed and secured. Two crosslinks are placed at the superior and inferior aspects of the construct to increase the overall stability (Fig. 37-5). These crosslinks aid rotational and translational stability. Closure of the diaphragm is performed and chest tubes inserted into the thoracotomy site. The patient postoperatively will require a rigid brace while upright for about 8 to 12 weeks, until bony fusion has occurred (Fig. 37-6). FIGURE 37-4 Axial CT scan shows bicortical placement of a vertebral body screw. FIGURE 37-5 Intraoperative photograph of a Kaneda screw-rod system in place. FIGURE 37-6 Lateral radiograph of an anterior screw-rod construct and cage. Complications of this procedure include worsening of neurologic status, dural tear with cerebrospinal fluid (CSF) leak, infection, and thoracic or retroperitoneal hematoma. Injury to the spleen, kidney, ureter, or vascular structures is possible. Postoperative atelectasis and pneumonia are also risks as well as an incisional hernia, dysesthesias, or pain along the thoracotomy incision. Graft expulsion or instrumentation failures are also risks anytime these devices are used. Newer oncologic treatment modalities have resulted in longer survival of cancer patients who are admitted with destruction of the thoracic and lumbar vertebral bodies by metastatic tumor.10 Thoracolumbar vertebrectomy is frequently indicated in such patients to decompress neural elements, to restore alignment, or to relieve local spinal or radicular pain, but it results in a destabilizing anterior spinal column defect. After such a procedure, reconstruction of the anterior and middle columns is required. The addition of anterior instrumentation to restore stability without posterior reconstruction has the benefit of reducing surgical morbidity when feasible. Anterior reconstruction can be performed using various graft materials such as bone graft, PMMA, and metal fusion cages. The type of graft may affect the stability of the surgical construct. Bone grafts in spine surgery are used to generate fusion and to provide structural support. The advantages of autogenous cortical-cancellous bone grafts are their immunocompatibility, their lack of potential for transferring disease, and their osteoinductive capability. Disadvantages in their use include a limited quantity of bone available, the potential for inadequate structural support in patients with osteoporosis, and significant donor-site morbidity. Complication rates as high as 25% have been reported after autogenous bone graft harvest including infection, increased blood loss, pain, and pelvic fracture.18,19 Cortical allografts in anterior spine reconstruction eliminate the potential disadvantages of autograft use. They provide a broad contact surface and good structural support for optimal load-bearing ability.20,21 Complications with the use of cortical allografts are cited to include nonunion, fractures, infection, and transmission of diseases.22,23 Human immunodeficiency virus (HIV) has been estimated to be <1/1,500,000 with allografts.24 In a study of radiographic union at allograft-host junctions in diaphyseal bone, Wunder et al19 noted a mean time for union to be 36 weeks (range, 22–54 weeks). McAfee et al25 found that the fusion rate for autogenous tricortical iliac crest used for anterior column reconstruction after trauma was 96%. Finkelstein and associates26 found an 81% fusion rate when cortical allograft was used in anterior column reconstruction after trauma. Autogenous rib is used for anterior strut grafting for many indications and has been described for use in metastatic disease.27 Problems with the lack of strength and cross-sectional area for contact have been noted. Vascularized rib autografts have been used successfully in kyphosis, but the advantages would not likely be seen in most cases of metastatic disease.28,29 Fibula strut grafting for anterior column defects and kyphosis is a well-established technique,30,31 but problems with late collapse and recurrent deformity have been reported in metastatic disease.32 Allograft bone (humerus, tibial, or femoral) is also an option for reconstruction. Allograft femur is a strong construct with good cross-sectional area that can evenly distribute the loads. Autogenous bone has been traditionally preferred for spinal reconstruction after vertebrectomy for trauma or reconstructions for benign tumors or degenerative conditions. Autograft use in the presence of metastatic spinal malignancy is less appropriate because radiation and chemotherapy may retard or prevent bony fusion, residual local tumor may invade the bone graft, and potential bone donor sites may be unsuitable because of tumor infiltration. When the radiation doses are limited to 1500 rad in an effort to heal bone grafts, the incidence of tumor recurrence is high.33-35 Additionally, anterior bone-grafting may not afford immediate spinal stability.33 Since autograft and allograft may not be the best reconstruction material in patients with spinal metastasis, other synthetic materials have been developed. PMMA has been used in a variety of surgical settings. The advantages of PMMA include the immediate ability to resist compression. In addition, although there is a minor ionization effect on tissue adjacent to radiopaque acrylic cement, no adverse effect from this phenomenon has been demonstrated.36,37 Experimental studies have shown that PMMA has excellent compressive strength and that irradiation causes no objective changes in the shear strength, compressibility, or durability.38–40 There is a possibility of vascular or neural damage occurring as a result of high temperatures developing near the surfaces of the polymerizing acrylic mass. Temperatures of up to 90°C have been recorded in hip replacements.41–43 However, insignificant temperature changes were noted experimentally in the subdural tissues when PMMA was applied directly to the lamina. They postulated that the constant flow of CSF functions to dissipate the heat. Scoville and associates34 first demonstrated the feasibility of anterior vertebral resection and replacement-stabilization using PMMA in dogs and later in a single patient with a lymphoma in the cervical spine. A variety of techniques have been described for vertebral body reconstruction with prosthetic materials. The most common method entails the use of Steinmann pins or Kirschner wires (K-wires) placed in adjacent vertebral bodies to secure an acrylic construct (Fig. 37-7).44,45 McAfee et al46 noted that stresses placed on the thoracic and lumbar spine are considerable, making isolated noninstrumented reconstruction prone to failure. Loosening and loss of fixation of anterior PMMA body replacement techniques have been reported. Sundaresan and coworkers45 observed a 5% fixation failure rate and recommended augmenting the construct with posterior fixation in junctional segments of the spine. In 1985, Siegal and Siegal16 described a technique of anchoring the PMMA with a threaded compression rod with a sacral hood embedded into the vertebral body above and below and PMMA placed over the whole construct. He reported that the construct dislodged in 3 of 20 patients. He felt this was due to faulty anchorage of the construct. To place the distraction forces directly in line with the center of the vertebral body, Harrington47 recommended placing a Knodt rod and hooks between the intact vertebral bodies. A notch is created within the center of the end plates of the intact vertebral bodies large enough to accept both a Knodt rod and the body of a hook. PMMA is then placed in the space and molded into position. In reporting his experience with treating 77 patients, Harrington observed that displacement of the PMMA occurred in 8% of patients.
Anterior Thoracolumbar Screw-Rod Instrumentation
 Indications for Anterior Decompression and Instrumentation
Indications for Anterior Decompression and Instrumentation
 Approach and Decompression
Approach and Decompression
 Anterior Interbody Reconstruction
Anterior Interbody Reconstruction
Bone
Polymethylmethacrylate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree