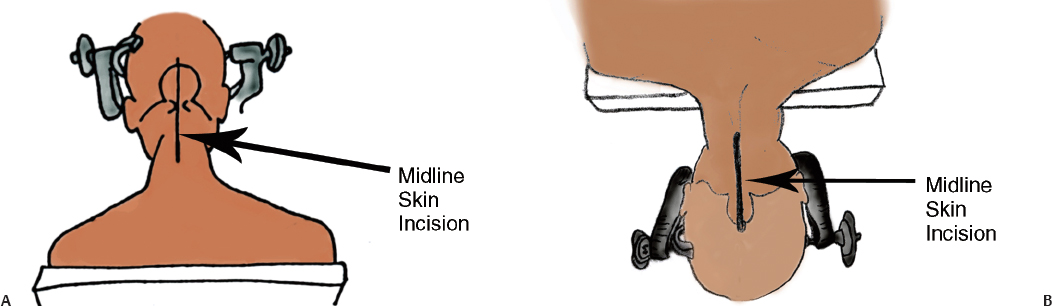
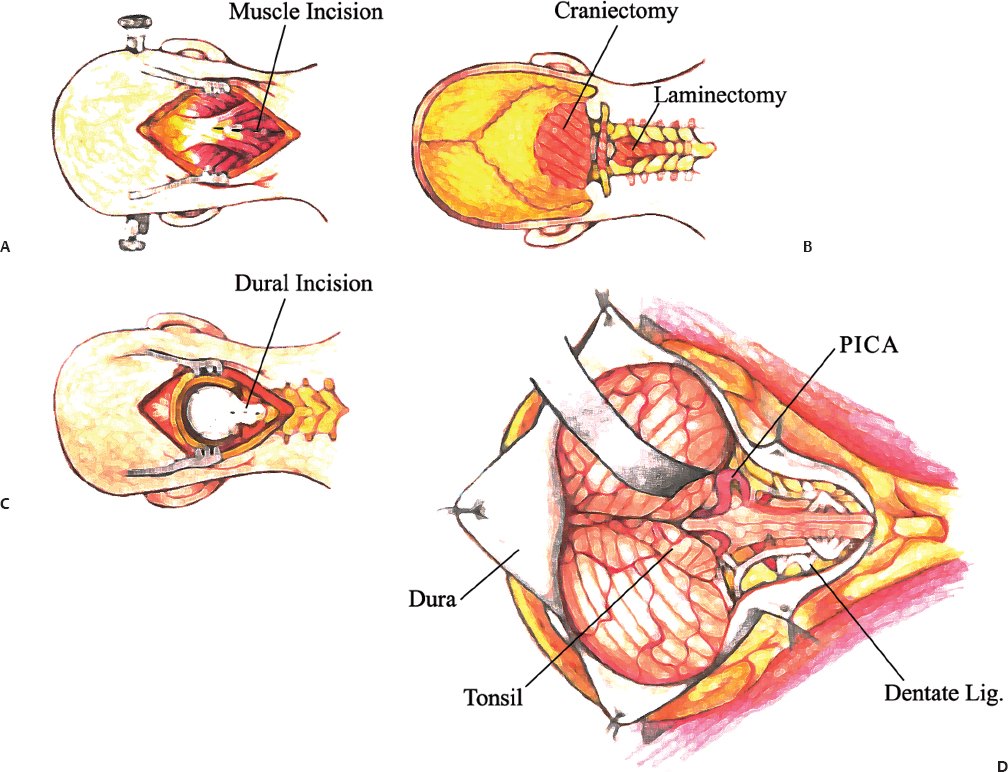
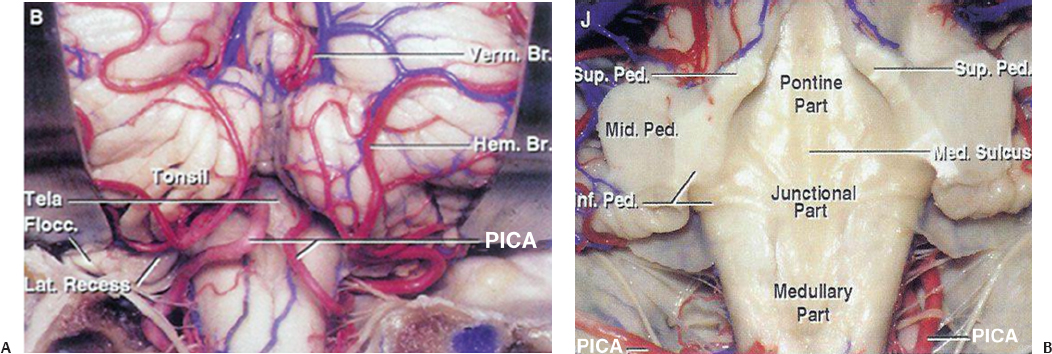
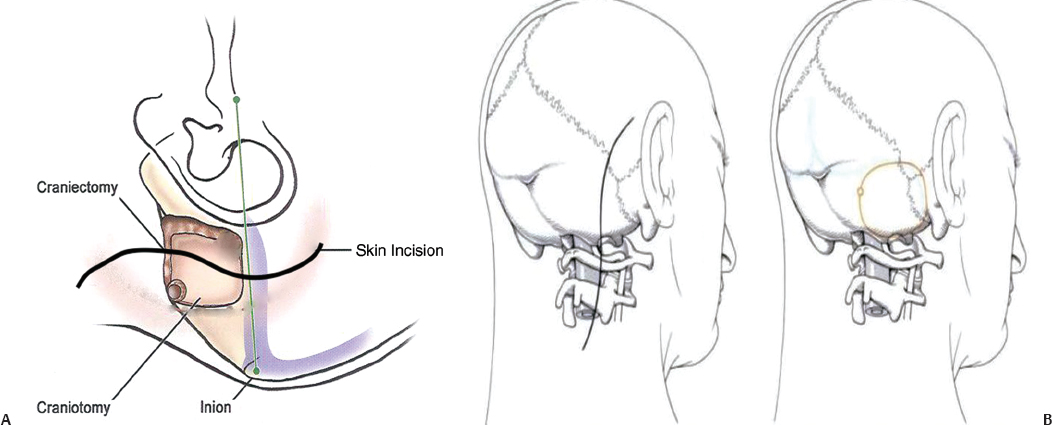
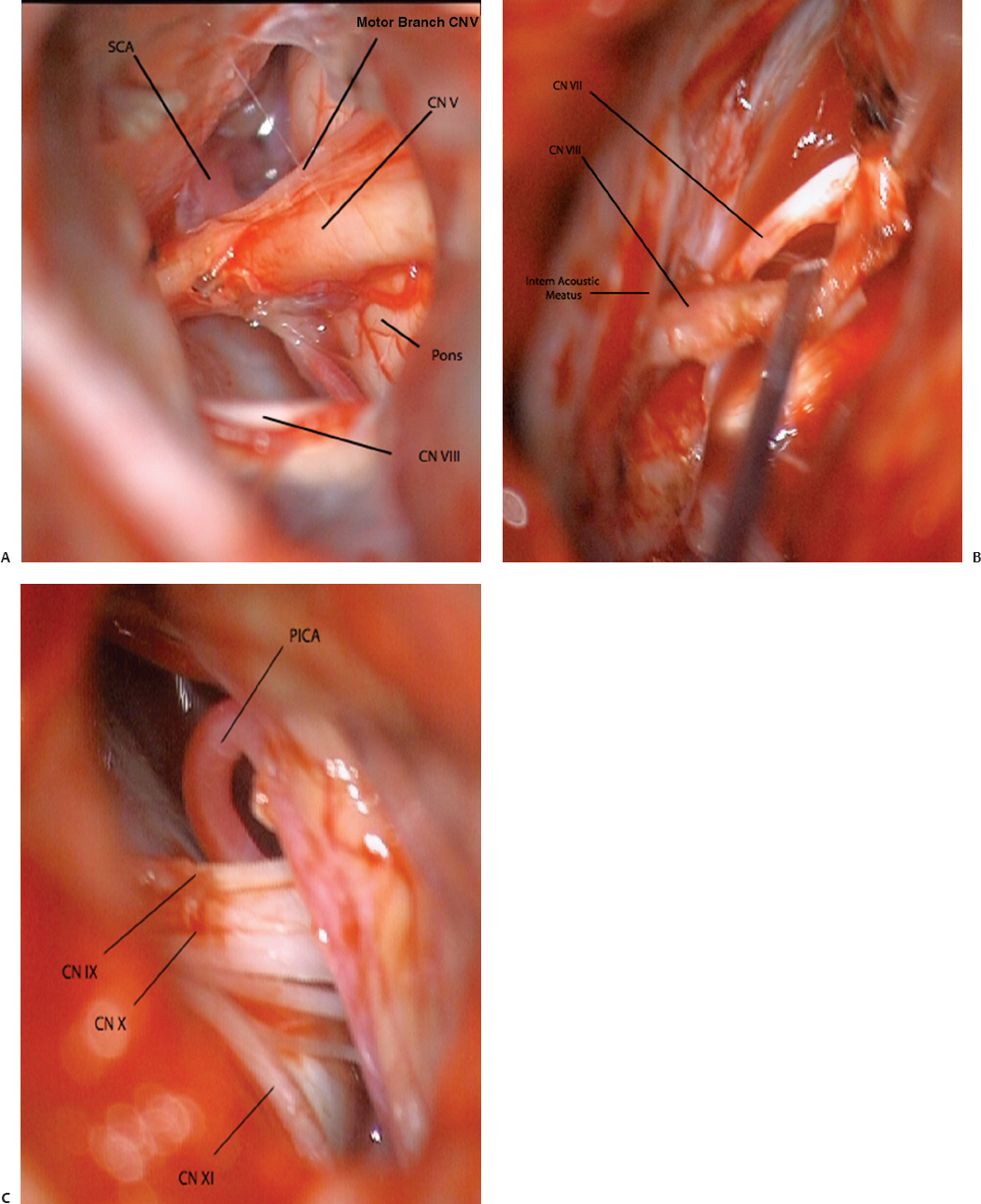
11 The neural structures found within the posterior fossa are responsible for some of the most critical functions in the central nervous system. The limited space that encompasses this compartment of the brain leaves little room for a mass lesion to form before it has the potential to become dramatically symptomatic. Brainstem compression, herniation, and death are all risks of mass lesions that occur in this location. The ability to safely treat pathology of the posterior fossa has significantly evolved over the course of the modern neurosurgical era.1 Advances in anesthesia, aseptic technique, neurologic localization, and technical tools and skills have allowed for safe and effective surgical treatment options to be developed. Two standard approaches are used to access the majority of posterior fossa pathology: the midline suboccipital approach and the retromastoid approach. Although some technical variability exists among neurosurgeons in regard to these procedures, the fundamental concepts remain the same. A thorough familiarity with these approaches is a critical skill for any neurosurgeon treating these lesions to possess. Preoperative considerations for posterior fossa surgeries should include a thorough history and physical examination, a cardiogram, urinalysis, chest x-ray, and laboratory tests that include a complete metabolic profile, complete blood count, and coagulation profile. All necessary neuroimaging should be obtained and carefully evaluated for vascular relationships to the lesion, ventricular size, and any distortion of normal anatomy. When appropriate, arrangements should be made for intraoperative neurophysiologic monitoring. Neuromonitoring is frequently done for lesions involving the cranial nerves. Providing quality neuroanesthesia for the patient is a critical component of a smooth intraoperative course during a posterior fossa surgery. Prior to the surgery there should be clear communication with the anesthesiologist regarding the administration of antibiotics, osmotic agents, and corticosteroids. Wide swings in blood pressure should be avoided during head-pin placement, and for the duration of the surgical procedure and emergence from anesthesia. In cases involving neurophysiologic monitoring of cranial nerves, the anesthesiologist needs to avoid giving any pharmaceutical agents, such as paralytics, that can interfere with the monitoring. The midline suboccipital approach can be used to access vascular, neoplastic, and developmental pathologies of the cerebellar hemispheres, vermis, fourth ventricle, brainstem, and foramen magnum.2 The surgical goals of this approach should include sufficient exposure, adequate decompression of the foramen magnum, avoidance of injury to the venous sinuses and structures adjacent to the fourth ventricle, and prevention of avoidable complications. The patient can be positioned in either the prone or sitting position. For the prone position, the head is placed in three-point pin fixation and the patient is positioned on chest rolls with padding at all pressure points. The prone position minimizes the risk of a venous air embolism and facilitates controlled release of cerebrospinal fluid (CSF). For the sitting position, the head is again secured in three-point pin fixation and flexed forward. This position facilitates increased venous drainage and subsequent lowering of the intracranial pressure, gravitational drainage of blood and CSF from the surgical field, and unrestricted ventilation. The risk of venous air emboli and a biomechanical disadvantage for the surgeon that can result in arm fatigue are disadvantages of this position. Precordial Dopplers and a central venous line in the right atrium should be placed prior to beginning the surgery in order to detect and evacuate any potential air emboli2,3 (Fig. 11.1). Fig. 11.1 Positioning and incision for midline suboccipital approach. (A) Sitting position. (B) Prone position. Several surface landmarks are important for planning the skin incision and craniotomy for the midline approach. The inion overlies the torcula, and the course of the transverse sinus can be approximated along the line between the inion and superior auditory meatus2–4 (Fig. 11.2). A line between the inion and the spinous process of C2 should be approximated. A standard vertical incision extends along this line from approximately 2 cm above the inion to the spinous process of C2. The incision can be shortened or lengthened depending on the exact nature of the pathology. Approximately 10 to 15 mm inferior to the superior nuchal line the bone thickness decreases dramatically. This occipital bone thickness increases again to 5 to 6 mm within 1 cm of the foramen magnum.5 The incision is made as described above, and deepened down to the bone along the midline avascular plane. The muscles are elevated from the underlying occipital bone in a subperiosteal fashion. The dorsal arch of C1 is exposed, taking care to remain medial to the vertebral arteries. Brisk bleeding from the surrounding venous plexus may serve as a warning sign that the vertebral artery is nearby. Self-retaining retractors are used to maintain exposure. A craniotomy is performed by placing two bur holes below the transverse sinus on either side of the midline septum. A craniotomy flap is turned and elevated. If necessary, a Midus Rex drill (Medtronic, Minneapolis, MN) with a B1 or C1 bit attachment can be used to score the edges of the craniotomy followed by a Kerrison rongeur to completely free the bone. Drilling and rongeurs should be used to create a wide opening at the foramen magnum, ensuring complete decompression of the brainstem. For lesions involving the foramen magnum and upper cervical spine, a laminectomy at C1 is performed. The dura is generally opened in a Y-shaped fashion with the straight limb of the Y extending down the midline to the region of the foramen magnum. The dural flaps are reflected superolaterally with sutures3,6 (Fig. 11.3). The cerebellar hemispheres will be visualized immediately. The arachnoid overlying the cisterna magna can be opened to drain CSF and allow for brain relaxation. The cerebellar tonsils should be identified as well as the posterior inferior cerebellar arteries (PICAs), noting that PICA anatomy can be variable. After freeing the arachnoid attachments, the tonsils can be gently retracted laterally, opening the foramen Magendie and leading to visualization of the floor of the fourth ventricle and cervicomedullary junction (Fig. 11.4). If the upper portion of the fourth ventricle and cerebral aqueduct need to be visualized, the cerebellomedullary fissure approach to the fourth ventricle may be used. The cerebellomedullary fissure separates the tonsils from the medulla oblongata. The PICA and the vein of the cerebellomedullary fissure course together through this space. To expose the fissure, the tonsils are retracted superolaterally. The lateral recesses of the fourth ventricle are exposed and can be followed to the foramen of Luschka, communicating with the cerebellopontine angle. The tela choroidea is identified where it forms the roof of the fourth ventricle. The tela is incised, facilitating entrance to the fourth ventricle.6–8 This approach enables the exposure of fourth ventricular tumors while avoiding division of the vermis and its associated complications. Image guidance can be of great utility for lesions involving the parenchyma of the cerebellum to minimize injury to normal parenchyma, especially the deep cerebellar nuclei. It is important to note that the cerebellar hemispheres relax and shift position after release of a significant amount of CSF. If using image-guided stereotaxy, it may benefit the surgeon to localize the lesion prior to releasing CSF. Fig. 11.2 (A) Soft tissue is divided in the midline and elevated laterally off the suboccipital bone. (B) Suboccipital craniotomy is performed with wide opening of the foramen magnum. A C1 laminectomy is performed for lesions lying at or below the foramen magnum. (C) The dural is opened in a Y-shaped fashion and reflected superolaterally. (D) The cerebellar hemispheres, tonsils, and posterior inferior cerebellar artery (PICA) can be visualized following dural opening. Fig. 11.3 (A) Posterior view of the cerebellum and PICA. (B) Floor of the fourth ventricle. Br., branch; Flocc., flocculus; Hem., hemisphere; Inf., inferior; Lat., lateral; Mid., middle; Ped., pedicle; PICA, posterior inferior cerebellar artery; Sup., superior; Verm., vermian. (Courtesy of Albert L. Rhoton, Jr., MD) Fig. 11.4 Lateral positioning for retromastoid approach. The head is flexed and tilted toward floor. Strict hemostasis should always be achieved prior to closure of the dura. The most common complication associated with posterior fossa surgery is leakage of CSF.9 To minimize the risk of either a leak or a pseudomeningocele, a watertight closure of the dural opening should be achieved. This may require using dural substitutes or autologous grafts and can be augmented by a dural sealant or fibrin glue. The Valsalva maneuver can be performed by the anesthesiologist to search for CSF communication through the dural closure. If there is no notable swelling in the posterior fossa at the time of closure, the bone flap should be secured into place with titanium microplates. The muscle, fascia, and skin should be closed in multiple layers to act as a secondary barrier against a CSF leak. During positioning of the patient, excessive turning of the head and neck can obstruct venous outflow and create elevated intracranial pressure. One potential vascular injury that can occur with this approach typically happens with aggressive lateral dissection of muscle off of the C1 lamina, resulting in injury to the vertebral artery as it courses across the posterior arch of the atlas. Another involves occluding the PICA while retracting the tonsils. The location of the PICA should always be noted prior to placing the retractors, and care should be taken not to compress or injure the artery.2,6 Surgical procedures involving the cerebellum and fourth ventricle carry a risk of injury to the surrounding neurologic structures. Although a significant amount of tissue in the cerebellar hemispheres can be sacrificed without any demonstrable loss of function, certain cerebellar structures are more symptomatic than others when compromised. Unilateral injury to the portion of the hemisphere lateral to the dentate nuclei may result in ataxia, hypotonia, and adiadochokinesia in the ipsilateral limbs. A direct injury to the dentate nucleus increases the severity and duration of these symptoms as well as results in an intention tremor with voluntary movements. Dysarthria can result from injury to the paravermian cerebellum. Injury to the middle cerebellar peduncle can lead to ipsilateral ataxia, dysmetria, and hypotonia. Dissection of tumors of the fourth ventricle can put the superior and inferior cerebellar peduncles at risk. Injury to the superior peduncle may result in intention tremor, dysmetria, and decomposition of movement, whereas injury to the inferior peduncle may result in ataxia and gait difficulties. Small lesions in the vermis are usually asymptomatic; however, injury to the portions containing vestibular fibers— namely the uvula, nodule, and flocculus—creates deficits of equilibrium, including truncal ataxia, staggering gait, head and trunk oscillation, and occasionally nystagmus. Cerebellar mutism is an occasional transient symptom seen in children after the removal of midline cerebellar lesions and is discussed in further detail below.7 Resection of tumors or other lesions involving the floor of the fourth ventricle can create problems with facial movement, lateral gaze, hypotension, apnea, speech and swallowing, and cough reflex. Postoperative swelling, bleeding, or arachnoid scarring can lead to obstruction of CSF flow and subsequent hydrocephalus. This can be diagnosed by neurologic examination and confirmed with computed tomography (CT) prior to placing an external ventricular drain. Aseptic meningitis can appear 5 to 7 days after the operation and is characterized by fever, photophobia, and nuchal rigidity. It is treated with corticosteroids; however, CSF cultures should be obtained and prophylactic antibiotics initiated until bacterial meningitis has been ruled out. The retromastoid approach can be used to access tumors of the cerebellopontine angle, vascular lesions in the posterior circulation, and pathologies involving cranial nerves V through XII. The surgical goals of this approach should include adequate exposure, avoidance of injury to the dura or venous sinuses, and prevention of avoidable complications.2,10 Fig. 11.5 Retromastoid approach: S-shaped incision (A,B-left), craniotomy (A,B-right), and relevant landmarks. We prefer the lateral position for this procedure, but other options include the sitting, three-quarter lateral, or supine position with the head maximally rotated. For the lateral position, an axillary roll is placed and all pressure points are padded prior to securing the patient to the operative table. The ipsilateral shoulder is pulled down to maximize the angle between the head and shoulder. After securing the head in three-point pin fixation, the sagittal suture is aligned parallel to the floor prior to gently flexing and laterally tilting the head toward the contralateral shoulder (Fig. 11.5). Several key landmarks should be identified. The line between the inion and the zygomatic root indicates the course of the transverse sinus. The digastric or mastoid groove indicates the position of the sigmoid sinus. The position of the asterion is variable in the cephalocaudal direction; however, it is located directly over the transverse-sigmoid junction in 74.4 to 86% of patients,11–13 and bur holes placed in this area risk damage to the underlying sinus. A retroauricular incision is made in either a curvilinear or lazy-S shape approximately 1 cm medial to the mastoid. The extent of the incision depends on the nature of the pathology, but typically it extends from the top of the pinna to the mastoid tip (Fig. 11.5). The soft tissue is elevated in subperiosteal fashion from the underlying bone, and a self-retaining retractor is placed to maintain exposure. A bur hole is made approximately 2 cm inferior to the asterion and 1 to 2 cm medial to the mastoid groove. A craniotomy flap is then turned, keeping the superolateral aspect at the edge of the transverse-sigmoid sinus junction. If necessary, further bone can be removed with the drill or rongeurs until the anterior margin of the bony opening reaches the medial edge of the sigmoid sinus. For pathology that involves the lower cranial nerves, the foramen magnum may need to be opened as well. The dura is opened in a K-shaped fashion and reflected with sutures. The cerebellum is then gently elevated until the arachnoid of the cisternal magna is visualized and opened, facilitating drainage of CSF and relaxation of the cerebellum. If necessary, a self-retaining retractor can be placed to gently retract the cerebellum downward to visualize the structures of the cerebellopontine angle. Angling the microscope toward the upper portion of the cerebellopontine angle facilitates visualization of the trigeminal nerve, superior cerebellar artery, and superior petrosal vein below the tentorium. The fourth cranial nerve may be seen along the medial edge of the tentorium behind the arachnoid plane. Moving the angle of the microscope slightly inferiorly reveals the cranial nerve VII/VIII complex entering the internal auditory canal, along with the anterior inferior cerebellar artery. Looking further inferiorly through the cranial opening reveals the lower cranial nerve complex (IX, X, and XI) along with the PICA, the vertebral artery, and the vertebrobasilar junction (Fig. 11.6). Strict hemostasis should be ensured prior to beginning the closure, and as with the midline approach, the dura should be closed in a watertight fashion to minimize the higher risk of CSF leakage. The Valsalva maneuver is performed by the anesthetist to inspect the dural closure. Dural sealants are appropriate if any communication through the dural closure is suspect. If a craniotomy has been performed, the bone flap should be secured into place. Patients who have undergone craniectomies are at risk for chronic craniectomy headaches, and a cranioplasty with bone substitute should be considered as part of the closure. The muscle, fascia, and skin should be closed in multiple layers to act as a secondary barrier against a CSF leak. Fig. 11.6 (A) Cranial nerve (CN) V and the superior cerebellar artery can be visualized at the superior aspect of the cerebellopontine angle. (B) The CN VII/VIII complex is visualized after resection of a small acoustic neuroma. CN VIII is retracted inferiorly, demonstrating CN VII. (C) The lower cranial nerves (IX, X, XI) and the posterior inferior cerebellar artery are visualized at the inferior aspect of the cerebello-pontine angle. Lacerations of the transverse or sigmoid sinus can lead to significant blood loss if not addressed expeditiously. When tamponading sinus bleeding, care must be taken not to occlude the vessel as this could lead to venous outflow obstruction and elevated intracranial pressure. If necessary, bony removal can be extended to repair a sinus laceration primarily. Any mastoid air cells that have been exposed during the opening should be sealed completely with bone wax to avoid CSF leakage. Excessive manipulation or retraction of the cerebellum can lead to posterior fossa swelling, obstruction of CSF outflow, and subsequent hydrocephalus. Cerebellar contusions can be caused by prolonged retraction or overly aggressive retraction in the setting of cerebellar swelling. If noted during the surgery, the contusion should be resected to avoid associated swelling in the postoperative period. Injury to the cranial nerves can have devastating consequences for the patient. Trigeminal and facial paralysis can lead to exposure keratopathy if not aggressively managed with lubricants. Facial paralysis can affect speech or lead to significant aesthetic consequences. Injury to cranial nerve VIII results in ipsilateral hearing loss. Injury to the lower cranial nerves (IX, X, and XI) can result in dysphagia and aspiration pneumonia. These injuries can generally be avoided with gentle retraction, careful microsurgical technique, gentle handling of the nerves and arteries, and neurophysiologic monitoring. The postoperative care varies depending on the patient’s pathology and any complications that occur; however, any patient who has undergone a surgical procedure in the posterior fossa should be monitored in an intensive care unit or neurologic step-down unit during the immediate postoperative period. Most neurosurgical patients are kept on prophylactic intravenous antibiotics for 24 hours. Corticosteroids should be continued from the operating room and weaned as appropriate. Blood pressure should be strictly controlled and antihypertensive medications used when necessary. Relevant neuroimaging should be obtained, including magnetic resonance imaging (MRI) within 24 hours following a tumor resection, angiography following treatment of vascular pathologies, and head CT to address any suspicion of hematoma formation, brain swelling, or development of hydrocephalus. Most importantly, the neurologic examination should be followed closely. Sedating medications should be avoided whenever possible and any deterioration in neurologic status should be evaluated immediately. Neurosurgical procedures addressing posterior fossa pathology carry a higher incidence of neurologic complication when compared with supratentorial procedures. Dubey et al9 report an overall complication rate of 31.8% in all posterior fossa surgeries, with CSF leaks occurring with the highest frequency, followed by meningitis, wound infection, and cranial neuropathies. The increased risk for complication in this area is likely related to the close proximity of critical neurovascular structures as well as the CSF fluid dynamics present in the region of the posterior fossa. Tumors in close proximity to the cranial nerves and surrounding critical vascular structures make complete surgical excision challenging, if not impossible. It is especially important when operating in this region of the central nervous system to keep in mind that a preserved quality of life holds greater value than total tumor excision. Cerebrospinal fluid leakage after a surgical approach is the most common complication of posterior fossa surgery, with a reported incidence of 4 to 17%.9,14,15 Factors that may predispose a patient to a higher risk of CSF leak include larger tumors, dural invasion of the tumor, abnormal underlying CSF hydrodynamics (i.e., hydrocephalus), intraventricular hemorrhage, brain edema, and elevated intracranial pressure. CSF leak following a retromastoid approach can drain through open mastoid air cells into the middle ear cavity, and then through the eustachian tube to present as CSF rhinorrhea. This type of leak has been referred to as paradoxical CSF rhinorrhea. Faithful wax occlusion of all mastoid air cells during the surgical approach successfully avoids this complication. Watertight dural closure should be the goal following any posterior fossa approach. If the dural edges cannot be completely approximated, the defects should be repaired with a form of autologous graft such as muscle or pericranium. Additionally, many forms of dural graft matrix are now produced and can also be used for dural defects. Dural sealants can be used to reinforce a closure and have demonstrated their value in the literature with significantly lower rates of associated CSF leakage.14,16 If CSF leakage through the wound occurs, conservative management should be initiated immediately, including wound resuturing, application of a pressure dressing, bed rest, and elevation of the patient’s head. Should the leak persist, the patient may need to be treated with lumbar drainage, or rarely surgical repair. Early recognition and treatment of known CSF leakage is important to avoid a secondary infection. The incidence of postoperative infection following posterior fossa surgery is far higher than infection following supratentorial craniotomies. This is likely due to the increased incidence of CSF leakage. Dubey et al9 reported a 13.6% incidence of all postoperative infections, including superficial, deep, and bone flap infections as well as meningitis. The majority of postoperative infections result from contamination of the sterile field during the surgical procedure. Other risk factors for this complication include persistent CSF leak, presence of a foreign body, prolonged surgical time, long-term steroid usage, diabetes, reoperation, and cytotoxic therapy.17,18 If meningitis occurs, early diagnosis and treatment with appropriate antibiotics is critical to avoid neurologic sequelae. To minimize the risk of postoperative infection, a broadspectrum antibiotic should be given prior to making the skin incision during the surgical procedure and for the immediate postoperative period. Standard sterile techniques, meticulous wound closure, and proper postoperative wound care minimize the risk of infection. Aseptic meningitis may mimic the clinical presentation of bacterial meningitis and demonstrate CSF pleocytosis, hypoglycemia, and hyperproteinemia. Unlike with bacterial meningitis, however, the cultures and Gram stain will be negative. Aseptic meningitis is a diagnosis of exclusion and is treated with steroids. Antibiotic treatment is unnecessary once bacterial meningitis has been ruled out. The etiology of this complication is unclear, but associations have been made between its development and the presence of blood or tumor cyst fluid in the basilar cisterns. It typically presents 4 to 7 days postoperatively, and there have been reports of resolution of symptoms following the closure of a concurrent pseudomeningocele. Cerebellar mutism is an uncommon but well-recognized complication after posterior fossa surgery; however, the precise mechanism and anatomic basis for this deficit remains unclear.19 This complication occurs most commonly in children after removal of tumors involving the cerebellar vermis. The patient is typically mute from day one to several days following surgery, and the symptom subsequently resolves over a period of weeks to months. Suspected causes include damage to the dentate nucleus, disruption of the dentatothalamocortical pathway, injury to the median structures of the cerebellum, and postoperative vasospasm of the cerebellar arteries.9 Avoiding this complication entails implementing surgical approaches that do not involve incision of the vermis, and instead use other techniques such as approach through the cerebellomedullary fissure for access to the fourth ventricle. Minimizing retraction on the cerebellar hemispheres may also help to avoid this deficit. The incidence of pseudomeningocele following posterior fossa surgery ranges from 15 to 28%.20 CSF leaks through the dural closure and collects under the skin and tissue planes, resulting in a fluctuating fluid collection. If the incision breaks down, a CSF leak will result. An enlarging pseudomeningocele may indicate the development of hydrocephalus and warrant CSF diversion.21 An increased risk for developing postoperative pseudomeningocele has been associated with posterior fossa craniectomy rather than craniotomy.22 Cranial nerve morbidity has been reported to occur in 4.8% of patients who undergo posterior fossa surgery.9 A postoperative cranial nerve deficit can result from nerve retraction, direct injury to the nerve, compromise of its blood supply, or postoperative vasospasm.9 Cranial nerves III, IV, and VI are more tolerant of manipulation and recover more easily than cranial nerves VII to XII. An injury to cranial nerves III, IV, and VI results in diplopia, and may require subsequent oculoplastic procedures. Damage to cranial nerve V is generally well tolerated with the exception of damage to V1, which can cause corneal ulcerations if the eye is not kept adequately lubricated. Cranial nerve VIII is extremely sensitive to manipulation and prone to injury. Profound hearing loss can occur as a result, even in the presence of anatomic preservation of the nerve. Deficits of the lower cranial nerves (IX, X, XI, XII) are less frequent but can be associated with significant deficits, including difficulty swallowing and inability to protect the airway, resulting in the need for feeding tube placement and tracheostomy to avoid aspiration pneumonia.9 Maintaining the continuity of the nerve during the surgical procedure provides the best chance of functional recovery should the nerve be otherwise injured. Intraoperative neurophysiologic monitoring of the cranial nerves enables the surgeon to minimize trauma to the nerves and maintain anatomic preservation. Several studies have demonstrated the importance of neurophysiologic monitoring in reducing the incidence of postoperative cranial nerve deficits.9,23,24 Postoperative hematoma has been reported to occur in 3% of posterior fossa surgeries.9 This complication is usually associated with resection of parenchymal lesions and inadequate tumor bed hemostasis. Patients typically present with depressed consciousness or focal neurologic deficit. Mass lesions are poorly tolerated in the posterior fossa, and early recognition with postoperative neuroimaging and surgical evacuation are critical to preventing permanent or devastating neurologic damage. Meticulous operative technique, control of coagulopathy, and tight perioperative blood pressure control minimize the occurrence of this complication. Posterior fossa edema is a postoperative complication that results from direct manipulation of the brain tissue. The amount of edema seen in the brain tissue is directly related to the length and force of tissue retraction that occurred during the surgery. If excessive retraction is used to obtain exposure, tissue damage will occur, leading to subsequent edema. Brain edema is a serious complication and can lead to neurologic deficits. Proper patient positioning, hyperventilation, high-dose corticosteroids, adequate bone removal, CSF drainage, diuretics, and intermittent retraction can help minimize the occurrence of this complication. Preservation of vasculature whenever possible and the use of limited coagulation and delicate handling of nervous tissue will also reduce the incidence of postoperative edema. The incidence of new-onset postoperative hydrocephalus following a posterior fossa surgery has been reported at 4.5%.9 The majority of these patients developed hydrocephalus as a result of another postoperative complication, such as edema, postoperative hematoma, infection, direct CSF obstruction, or impaired CSF absorption after spillage of blood into the CSF cisterns and subarachnoid space.25,26 Prior cranial surgery or prior radiation may also increase the risk of developing this complication. Postoperative hydrocephalus can present with headache, nausea/vomiting, gait disturbance, abducens nerve palsy, or an enlarging cranium and bulging fontanel in young children.9 The development of hydrocephalus may manifest as CSF leakage, and should always be suspected in the setting of a leaking wound. This complication can be minimized by preventing other forms of postoperative complications such as hematoma, edema, and infection. The treatment is CSF diversion via shunting procedure. The basic approaches to the posterior fossa can be used to treat a wide range of pathologies. The frequency of complications is dependent on the type, size, and location of the pathologic lesion as well as the careful handling of neurovascular structures and the fastidious exposure and closure of the surgical wound. Meticulous microsurgical technique is critical to keep morbidity to a minimum. Learning the nuances and pitfalls associated with these procedures enables the neurosurgeon to treat posterior fossa pathology safely and effectively. 1. Shen T, Friedman RA, Brackmann DE, et al. The evolution of surgical approaches for posterior fossa meningiomas. Otol Neurotol 2004;25: 394–397 PubMed 2. Sarma S, Fossett D. Midline and paramedian suboccipital approaches. In: Fossett D, Caputy A, eds. Operative Neurosurgical Anatomy. New York: Thieme, 2002:86–89. 3. Rhoton AL Jr. Cerebellum and fourth ventricle. Neurosurgery 2000;47(3, Suppl):S7–S27 PubMed 4. Day JD, Kellogg JX, Tschabitscher M, Fukushima T. Surface and superficial surgical anatomy of the posterolateral cranial base: significance for surgical planning and approach. Neurosurgery 1996;38:1079–1083, discussion 1083–1084 PubMed 5. O’Brien MF. Surgical anatomy of the cervical spine. In: DeWald RL, ed. Spinal Deformities. The Comprehensive Text. New York: Thieme, 2003: 33–45 6. Jallo G, Goh K, Epstein F. Brain stem and cervicomedullary tumors. In: Sekhar LN, Fessler RG, eds. Atlas of Neurosurgical Techniques. New York: Thieme, 2006:457–465 7. Rhoton AL. The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. In: Rhoton’s Anatomy, Part 1. Schaumburg. IL: Lippincott Williams & Wilkins, 2003:525–561 8. Matsushima T, Inoue T, Inamura T, Natori Y, Ikezaki K, Fukui M. Transcerebellomedullary fissure approach with special reference to methods of dissecting the fissure. J Neurosurg 2001;94:257–264 PubMed 9. Dubey A, Sung WS, Shaya M, et al. Complications of posterior cranial fossa surgery—an institutional experience of 500 patients. Surg Neurol 2009;72:369–375 PubMed 10. Sarma S, Avci E, Fossett D. Retrosigmoid craniotomy. In: Fossett D, Caputy A, eds. Operative Neurosurgical Anatomy. New York: Thieme, 2002: 81–85 11. Day JD, Tschabitscher M. Anatomic position of the asterion. Neurosurgery 1998;42:198–199 PubMed 12. Avci E, Kocaogullar Y, Fossett D, Caputy A. Lateral posterior fossa venous sinus relationships to surface landmarks. Surg Neurol 2003;59: 392–397, discussion 397 PubMed 13. Ucerler H, Govsa F. Asterion as a surgical landmark for lateral cranial base approaches. J Craniomaxillofac Surg 2006;34:415–420 PubMed 14. Than KD, Baird CJ, Olivi A. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery 2008;63(1, Suppl 1):ONS182–ONS186, discussion ONS186–ONS187 PubMed 15. Nutik SL, Korol HW. Cerebrospinal fluid leak after acoustic neuroma surgery. Surg Neurol 1995;43:553–556, discussion 556–557 PubMed 16. Sawamura Y, Asaoka K, Terasaka S, Tada M, Uchida T. Evaluation of application techniques of fibrin sealant to prevent cerebrospinal fluid leakage: a new device for the application of aerosolized fibrin glue. Neurosurgery 1999;44:332–337 PubMed 17. Bennett M, Haynes DS. Surgical approaches and complications in the removal of vestibular schwannomas. Otolaryngol Clin North Am 2007; 40:589–609, ix–x PubMed 18. Narotam PK, van Dellen JR, du Trevou MD, Gouws E. Operative sepsis in neurosurgery: a method of classifying surgical cases. Neurosurgery 1994;34:409–415, discussion 415–416 PubMed 19. Kotil K, Eras M, Akçetin M, Bilge T. Cerebellar mutism following posterior fossa tumor resection in children. Turk Neurosurg 2008;18:89–94 PubMed 20. Manley GT, Dillon W. Acute posterior fossa syndrome following lumbar drainage for treatment of suboccipital pseudomeningocele. Report of three cases. J Neurosurg 2000;92:469–474 PubMed 21. Culley DJ, Berger MS, Shaw D, Geyer R. An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery 1994;34:402–407, discussion 407–408 PubMed 22. Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. Surgical procedures for posterior fossa tumors in children: does craniotomy lead to fewer complications than craniectomy? J Neurosurg 2002; 97:821–826 PubMed 23. Kartush JM, Larouere MJ, Graham MD, Bouchard KR, Audet BV. Intraoperative cranial nerve monitoring during posterior skull base surgery. Skull Base Surg 1991;1:85–92 PubMed 24. Schlake HP, Goldbrunner RH, Milewski C, et al. Intra-operative electromyographic monitoring of the lower cranial motor nerves (LCN IX-XII) in skull base surgery. Clin Neurol Neurosurg 2001;103:72–82 PubMed 25. DeLand FH, James AE Jr, Ladd DJ, Konigsmark BW. Normal pressure hydrocephalus: a histologic study. Am J Clin Pathol 1972;58:58–63 PubMed 26. Duong DH, O’malley S, Sekhar LN, Wright DG. Postoperative hydrocephalus in cranial base surgery. Skull Base Surg 2000;10:197–200 PubMed
Basic Concepts in Posterior Fossa Surgery
♦ Preoperative Management
♦ The Midline Suboccipital Approach
Positioning
Landmarks
Surgical Technique
Cerebellomedullary Fissure Approach to the Fourth Ventricle
Closure
Pitfalls and Complications
♦ The Retromastoid Approach
Positioning and Incision
Landmarks
Surgical Technique
Closure
Pitfalls and Complications
Postoperative Care
♦ Complications and Their Avoidance in Posterior Fossa Surgery
Cerebrospinal Fluid Leaks
Wound Infection and Meningitis
Aseptic Meningitis
Cerebellar Mutism
Pseudomeningocele
Cranial Nerve Palsies
Postoperative Hematoma
Posterior Fossa Edema
Hydrocephalus
♦ Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








