CHAPTER 213 Birth Brachial Plexus Injury
Pediatric obstetric brachial plexus palsy results from injury to one or more cervical and thoracic nerve roots (C5-T1) before, during, or after the birth process. The incidence of this injury has been estimated to range from 0.19 to 2.5 per 1000 live births1,2 but has declined significantly over the past century as a result of early recognition of risk factors, improved obstetric technique, and an increased rate of cesarean section.1,3–5 A recent 3-year review of more than 11 million births in the United States revealed an incidence of 1.51 per 1000 live births.6
Most injuries are mild and improve after conservative management,3,7 and some authors have concluded that complete recovery occurs in 70% to 95% of cases.3,5,8,9 However, several current studies have portrayed a more dismal spontaneous recovery rate, with close to 60% of patients experiencing permanent impairment.10,11
In the past, injuries that failed to improve were thought not to be amenable to surgical treatment.12 Although many controversies remain, it is clear that with proper patient selection, timing of surgical intervention, and implementation of microsurgical techniques, pediatric brachial plexus palsies can be treated surgically.
Surgical repair will result in improved function of the arm, not return of complete motor strength. Even though only a few hundred axons seem to be sufficient to reinnervate poorly differentiated muscles such as the biceps, thousands are required to restore the delicate balance of the intrinsics of the hand.13 It is thus unlikely that the hand will regain complete motor function in patients with severe brachial plexus injury.
History
Smellie, a London obstetrician, is credited with the first medical description of a brachial plexus injury in 1768.14 In 1872, Duchenne in his book Traite de l’Eelectrisation Localisee et de son Application à la Pathologie et à la Thérapeutique described traction during birth as the mechanism of injury.15 Erb in 1874 reported that weakness of the deltoid, biceps, coracobrachialis, and brachioradialis muscles was caused by disruption of the C5 and C6 nerve roots.16 The location where the C5 and C6 nerve roots join to form the upper trunk is thus called Erb’s point. The much less common lower brachial plexus palsy (C8-T1) was described by Klumpke in 1885.17
Surgery for obstetric brachial plexus injury was introduced in 1903 by Kennedy, who reported on three patients. At 2 months of age, his patients underwent resection of C5-6 neuromas with direct suturing of the ends, and favorable results were achieved.18 Additional reports during the early 1900s resulted in many surgeons advocating early brachial plexus surgery in children who did not improve spontaneously.19–21
In 1925, Sever concluded that there was no difference in outcome between surgically treated and conservatively managed patients after examining 1100 cases of obstetric brachial plexus palsy.12 Sever’s results, coupled with the relatively high morbidity and mortality rates associated with surgery during the first part of the century, led to almost complete abandonment of brachial plexus surgery for nearly 5 decades.
With the advent of microsurgical techniques, the operating microscope, and modern pediatric anesthesia, resurgence of interest in the surgical treatment of brachial plexus injuries ensued in the 1970s. The reports of Gilbert and Tassin demonstrating favorable outcomes after direct repair of obstetric brachial plexus injury22,23 are largely responsible for the increasing use of surgical treatment in the past 3 decades.
Pathophysiology
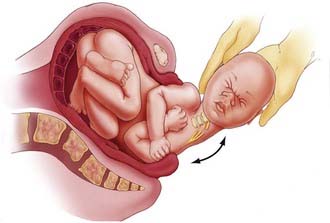
The traumatic origin of birth brachial plexus injury is considered to be a result of excessive traction applied to nerves secondary to shoulder dystocia, the use of extreme or improper traction, and hyperextension of the arms during breech delivery.7 Damage to the upper roots of the brachial plexus usually occurs during vertex delivery when shoulder dystocia necessitates excessive lateral flexion of the neck with the arm in adduction to free the shoulder from the pubic arch (Fig. 213-1). The right arm is involved more frequently because of the more common left occiput–anterior position of the descending fetus.24 During breech deliveries, the upper roots are also more commonly affected.25,26 One percent of obstetric palsies have occurred after cesarean section.27
A number of studies have identified risk factors for these injuries, including high birth weight (>4 kg), shoulder dystocia, prolonged labor, primiparity, grand multiparity, instrument deliveries, breech presentation, siblings with obstetric palsy, maternal age (>35 years), maternal pelvic anatomy, and maternal gestational diabetes.28–33 Although some patients with birth brachial plexus injury will have one or more risk factors, other will not have any. The last point has led many to raise questions regarding the etiology of this condition.
There appears to be a lack of evidence of identifiable risk factors for neonatal brachial plexus palsy.34–38 Although shoulder dystocia is the most common risk factor identified, only 17% of patients with neonatal brachial plexus palsy in the largest series to date had this risk factor.6 The same study found that only 46% of infants with brachial plexus palsy had one or more identifiable risk factors.6 An example of patients born without risk factors include uncomplicated vaginal deliveries of infants with smaller than average birth weight.39,40 These reports demonstrate that not all permanent brachial plexus injury is due to physician traction.39 Some authors speculate that individual infants have inherent biologic variability in susceptibility to brachial plexus palsy.41
Bilateral injuries are rare (4%) and occur more commonly with breech presentation.25,42 Neonatal brachial plexus injuries can be associated with clavicle and humerus fractures, facial nerve injury, cephalohematoma, and torticollis, but these conditions do not appear to portend a worse prognosis.
Natural History
As mentioned previously, recent studies indicate a good prognosis for patients, with 70% to 100% achieving complete spontaneous recovery (Table 213-1).3,5,9,43–46 Over the past 3 to 4 decades, the rate of recovery has been used to predict which patients will have full spontaneous recovery and which will have incomplete recovery, thus identifying patients who could benefit from surgical reconstruction of the brachial plexus. Poor prognostic features after obstetric brachial plexus injury include total plexopathy and lower plexus injury, typically characterized by Horner’s syndrome and increased root avulsion.26,45,47,48 These injuries usually result in incomplete recovery. In general, injuries to the upper plexus are thought to be less severe and carry a better prognosis.
Infants who have complete recovery of motor function will show improvement in strength as early as 2 weeks of age, with normalization of strength complete by 12 to 18 months.49 Assessment of strength in the biceps, triceps, and deltoid muscle groups by 6 months of age was an excellent predictor of outcome in our series of patients who experienced complete recovery.46 Each child recovered to 3/5 strength in all three muscle groups by 5.5 months. Infants who show no signs of recovery and have a flaccid upper extremity at 2 months of age have suffered severe total brachial plexus palsy, and their long-term outcome is poor.21,22,50–52 Some investigators have noted that if an infant is to have functional use of an extremity, some recovery must be seen by 4 months of age.8,9,26,49 We found that patients who experience persistent moderate weakness (less than antigravity strength of the biceps at 6 months of age) have unsatisfactory outcomes with observation.46 This study and others have concluded that biceps muscle function at the end of the third month is the most important indicator of functional recovery of the affected limb.45,46,53 Michelow and colleagues reported that favorable outcome was correctly predicted by biceps function at 3 months in 87% of patients.45 The prediction rate was improved to 95% if biceps function along with extensor function of the elbow, wrist, thumb, or finger was used in the clinical assessment. Tassin’s study monitored 20 patients prospectively and found that if infants do not have biceps function by 3 months of age, limited shoulder movement is a probable consequence.53 Recently, this particular finding has come into question inasmuch as Smith and coauthors reported good long-term shoulder function in patients with absent biceps function.54 However, the prognostic value of absence of biceps function on improvement in shoulder function has yet to be determined.
Clinical Findings
Brachial plexus injury patterns vary and can be classified by two distinct schemes: anatomic localization and severity of pathology. Anatomic localization is used most by clinicians and allows surgical planning. Pathologic severity is difficult to apply clinically and is based on Seddon and colleagues’ original work, which defined the terms neurapraxia (stretch injury without axonal disruption), axonotmesis (physical disruption of axons or nerve fascicles), and neurotmesis (complete disruption of a nerve trunk or root).55
There are four major anatomic patterns of injury. The first is a purely upper brachial plexus lesion involving only C5 and C6; it results in weakness of the deltoid and biceps, with sparing of the triceps and distal musculature. It has been suggested that such patients typically demonstrate good spontaneous recovery.56,57 The second and most common form is classic Erb’s palsy involving C5-7. This pattern is characterized by an extended arm, internally rotated shoulder, volar-flexed wrist, and extended fingers resulting in the “waiter’s tip” posture. The third type of injury is a total plexopathy involving C5-T1, which accounts for 20% of obstetric palsies.57 Physical examination reveals a flaccid, insensate arm with marbled skin secondary to vasomotor disturbance. Damage to the spinal cord may have occurred. The last type of injury pattern seen is Klumpke’s palsy, which involves only the lower (C8-T1) brachial plexus. Clinically, it is manifested as an inability to pronate the forearm, hand paralysis with a “clawhand” posture, and Horner’s syndrome. Isolated lower brachial plexus injury is exceedingly rare and reportedly accounts for less than 1% of obstetric palsies.56 We have not encountered such a case in our own patients. Patients with injury to the lower brachial plexus are less likely to recover useful function of the arm with conservative management than are those who suffer upper brachial plexus injury.
Other causes of arm immobility or paralysis, both reversible and irreversible, should be considered in the differential diagnosis, including epiphyseal separation of the humeral head, fracture of the clavicle or humerus, septic arthritis of the upper limb, birth-related spinal cord injury, and congenital varicella of the upper limb.58
Patient Evaluation
Since 1991, evaluation of patients with obstetric brachial plexus palsy at our institution has been performed in a multidisciplinary brachial plexus clinic by a team consisting of a pediatric neurosurgeon, pediatric neurologist, orthopedic surgeon, physical and occupational therapists, electrophysiologist, and nurse coordinator. A detailed obstetric and birth history is taken, with attention to the predisposing factors discussed previously. Associated injuries such as rib, spinal, clavicle, or humeral fractures are noted on plain radiographs. Clinically observed asymmetry of chest wall expansion and chest x-ray evidence of an elevated hemidiaphragm denote phrenic nerve injury. The patient is assessed for evidence of facial paresis or Horner’s syndrome. Passive range of motion of the involved arm, especially the shoulder, is evaluated. Motor strength is assessed with the simplified British Medical Council scale for muscle testing (0 to 5 scale). The Mallet score, which documents functional changes in the shoulder and arm, requires patient cooperation and is used additionally in patients older than 2 years.59 Sensory examination is difficult, although reaction to pinch is helpful. Sensory loss is sometimes evidenced by self-mutilation (i.e., biting) of fingers.
The infant is re-examined at 4 weeks of age. Frequently, recovery is complete or nearly complete, which signifies a neurapraxic injury. At this point, if there is still total palsy associated with Horner’s sign, the outlook is poor, and parents are informed of the possible need for surgical intervention. If hand function is improving without evidence of shoulder recovery, the patient is managed conservatively because there is still opportunity for recovery.60 Patients are examined on a monthly basis. Infants who make progressive recovery and have antigravity or greater than antigravity strength in the biceps, triceps, or deltoid muscles by 3 to 4 months of age continue to be monitored expectantly.46 Infants displaying less than antigravity strength in each of these muscles are referred for neuroimaging and electrophysiologic studies in anticipation of surgery. If the deltoid, biceps, or triceps muscle still has less than antigravity strength at 6 months of age, we recommend brachial plexus surgery (Table 213-2). Braces are no longer advocated because of the propensity for contracture.25
TABLE 213-2 Indications for Surgical Intervention
| Flaccid upper extremity (≥2 months of age) |
| Less than 3/5 motor strength in the deltoid, biceps, and triceps, with the presence of Horner’s syndrome on physical examination or pseudomeningocele on magnetic resonance imaging (≥3 months of age) |
| Less than 3/5 strength in the deltoid, biceps, and triceps in the affected extremity (≥6 months of age) |
When surgery is being considered, electromyography (EMG) with nerve conduction studies is delayed until the third month. If an intrauterine lesion is suspected, EMG is performed within a week after delivery to document denervation.61,62 EMG can provide useful information on the extent and distribution of brachial plexus injury and the recovery pattern. In addition, nerve root avulsion can be confirmed by normal sensory conduction in the presence of severe motor weakness. The usefulness of EMG in postoperative follow-up of patients is unclear. In infants, it takes only a small number of intact axons to provoke an electrical response that may not be associated with clinical recovery.
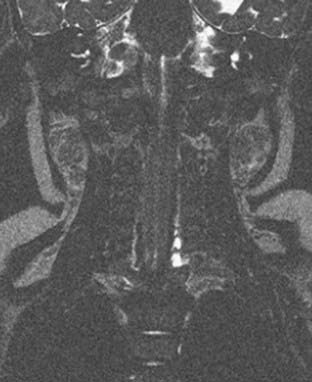
Preoperative cervical magnetic resonance imaging (MRI) evaluates nerve root avulsion.63–65 The presence of pseudomeningoceles has been associated with nerve root avulsion; however, this finding is not pathognomonic and should instead be considered a sign of root damage inasmuch as 15% of pseudomeningoceles are not associated with complete nerve root avulsion.66 In addition, 20% of avulsed nerve roots may not have an associated pseudomeningocele.67 We use fast spin echo MRI for preoperative neuroimaging because it is less invasive and provides data comparable to that of computed tomographic myelography for operative planning (Fig. 213-2).47 MRI does not clearly delineate nerve roots within the pseudomeningocele. In cases of total palsy, MRI allows imaging of the spinal cord.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree