22 A large number of neoplasms, both benign and malignant and also primary or secondary, can occur in the varied anatomic regions covered in this chapter, with management of spinal metastasis covered in detail in Chapters 19 and 24. Primary lesions of the conus and cauda equina can arise from cellular elements of the central nervous system (CNS) or peripheral nervous system (PNS) and from vestigial embryonic structures such as the filum terminale. Corresponding clinical symptoms are often intriguing, with a mixture of lower and upper motor nerve signs, though the former usually prevails, with bowel and bladder symptoms and signs being critical. Paraspinal lesions can have both an intradural and extradural component, again with a mixture of upper and lower motor symptoms and signs. Approaches to these lesions need to account not only for decompressing the intradural lesion and sparing critical roots or trunks of origin but also for spinal stability that may be compromised due to tumor growth or the required bony exposure for safe tumor removal during surgery. Peripheral nerve tumors also can be subdivided into benign and malignant and also primary or the rare secondary tumors that abut or sometimes directly infiltrate the nerve. The cancer predisposition syndrome, neurofibromatosis type 1 (NF1), is commonly associated with peripheral nerve tumors, and hence the clinical and molecular diagnosis of this syndrome is reviewed. Malignant peripheral nerve sheath tumors (MPNSTs), in the context of NF1 or sporadic patients, are rare tumors that need to be managed in specialized centers as outlined below. In most of the neurosurgical literature these tumors are dealt with from a pathologic rather than an anatomic or topographic point of view. Freeman and Cahill1 described these tumors from a topographic viewpoint. Tumors in this region may arise from cauda equina or conus medullaris neural tissue (including the coverings) or from the adjoining region (Table 22-2). Although most of the tumors of cauda equina are benign, they are not always easy to manage. The result is dependent on “total” removal of these lesions.
Cauda Equina, Paraspinal, and Peripheral Nerve Tumors
 Tumors Primarily of the Conus Medullaris or Cauda Equina
Tumors Primarily of the Conus Medullaris or Cauda Equina
Clinical Features (Table 22-1)
| Symptoms/signs | Cauda equina | Conus medullaris |
| Pain | Severe and radicular | Less severe, mainly back versus radicular |
| Hypoesthesia | Radicular sensory loss (saddle hypoesthesia) | Generally restricted to perianal region |
| Motor deficits | Asymmetric areflexic paraplegia | Distal paresis of lower limbs |
| Sphincters | Urinary retention | Urinary retention plus atonic anal sphincter |
| Sexual dysfunction | Rarely impotence | Frequently impotence |
| Origin | Type |
| Nerve sheath | Neurofibromas, schwannomas, MPNSTs |
| Meninges | Meningiomas |
| Glial cells | Astrocytomas, myxopapillary ependymomas, oligodendrogliomas |
| Metastasis | Epidural, intradural |
| Bone | Osteoid osteomas, osteoblastomas, osteochondromas, giant cell tumors, aneurysmal bone cysts, eosinophilic granulomas, osteosarcomas, chondrosarcomas, Ewing’s sarcomas, multiple myelomas |
| Blood vessel elements | Hemangioblastomas, hemangiomas |
| Others | Paragangliomas, chordomas, lipomas, epidermoid cysts, dermoid cysts, teratomas |
In general, neoplasms of the conus medullaris manifest neurologic symptoms earlier than those in the cauda equina, due to the more tolerant peripheral nerve roots in the latter, compared with pathologies arising from the spinal cord in the conus medullaris. This diagnostic delay is mainly dependent on the biologic growth rate of the tumor, with long-standing symptoms for relatively indolent primary tumors such as ependymomas, schwannomas, neurofibromas, and paragangliomas, in contrast to a short history for metastatic tumors. The characteristic physical signs of lesions in the region of cauda equina and neighboring conus medullaris are hypotonic bladder and rectal sphincters, pain, and loss of sensation in a saddle distribution around the perineum; however, subtle differences in the clinical symptoms that preferentially affect the cauda equina versus conus medullaris exist and are listed in Table 22-1, although the overwhelming majority of neoplasms in this region presents with a mixture of signs and symptoms. Though night and rest pain are nonspecific common features of many spinal tumors, differentiating neoplasms from the more prevalent degenerative causes of spinal pain, cauda equina tumors may present with long-standing persistent discrete dermatomal or radicular pain due to impairment of function of the single nerve root of origin. In a large series of nerve sheath tumors and ependymomas of the cauda equina, the most common symptom was radicular pain, whereas saddle anesthesia was relatively rare.2 In comparison, neoplasms in the region of conus medullaris predominantly affect the parasympathetic innervation of the bladder, bowel, and sexual organs and tend to present with these signs and symptoms.
Radiologic Diagnosis
Imaging studies, although not diagnostic for the variable pathologies of neoplasms that occur in this region, can often be highly suggestive. Plain x-rays are a useful but often forgotten first-line imaging study to delineate bony outlines, pathologic fractures, and remodeling. Computed tomography (CT) is the imaging modality of choice for definition of bony anatomy, with magnetic resonance imaging (MRI) the investigation of choice to delineate the tumor and adjacent soft tissue structures. These two prime imaging modalities are sometimes supplemented by angiography and bone scans, depending on the anatomic location and most likely type of tumor.
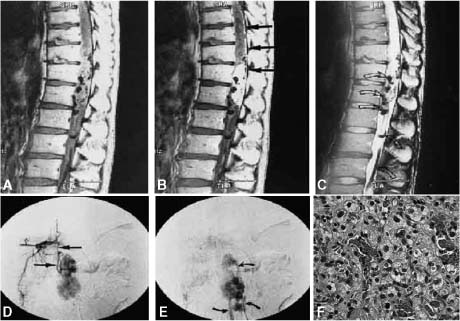
Pathologic examination is required for definitive diagnosis, with tissue usually obtained during surgical removal, though occasional preliminary CT-guided biopsy may be indicated. MRI characteristics suggestive of common lesions in the conus and cauda equina include the round and well-marginated schwannomas, which are isoor slightly hypointense on T1-weighted images and hyperintense on T2-weighted images, with gadolinium enhancement. Schwannomas (Fig. 22-1A) and neurofibromas (Fig. 22-1B) arising from the peripheral nerve roots of the cauda equina region may be totally intracanalicular or grow to varying extents through the neural foramina into the extraspinal space, giving rise to the so-called dumbbell tumors. When erosion of the pedicle and scalloping of the posterior vertebral body are present in association with an intracanalicular neoplasm, the diagnosis of either a schwannoma or neurofibroma is highly suggestive. Differentiation between schwannoma and neurofibroma radiologically is usually hard, except in patients with NF1, where they are always neurofibromas. Schwannomas tend to be more discrete and homogenously enhancing compared with neurofibromas, though many overlapping radiologic features between these two common neoplasms in this region exists. Neurofibromas sometimes display a low signal area within the mass, which is probably collagen stroma. In comparison, paragangliomas (Fig. 22-1C) are hyper- to isointense on T1-weighted MRI and, isointense on the proton density sequence, with bright enhancement. These tumors are highly vascular, as suggested by MRI flow voids, and an intense tumor blush if spinal angiography, which is rarely indicated, is undertaken. Paragangliomas are also positive for the rarely used MIBG (metaiodobenzylguanidine) scan, which can differentiate between these tumors and other common neoplasms of the conus or cauda equina.
Meningiomas are another group of common tumors in this region, with similar MRI features to nerve sheath tumors described above, except for a few subtle differences (Fig. 22-1D). In contrast to schwannomas and neurofibromas, meningiomas rarely extend through the neural foramina and tend to be more posterolateral in location, with occasional enhancement of adjacent dura, the so-called dural tail. Hemangioblastomas (Fig. 22-1E) are also a relatively common lesion with characteristic but not pathognomonic MRI features. These neoplasms, which for the most part have to be managed like spinal arteriovascular malformations, are hypo- to isointense on T1- and hyperintense on T2-weighted MRI, with intense gadolinium enhancement and flow voids associated with the mural nodules.
FIGURE 22-1 (A) Intracanalicular schwannoma involving the cauda equina (T1 and gadolinium). Hematoxylin and eosin (H&E) stain (100×) of a typical schwannoma with S-100 positive spindle cells with elongated nuclei. (B) Dumbbell neurofibroma arising from the lower thoracic spinal canal with enlargement of the foramen and displacement of the thecal sac, in a non-neurofibromatosis type 1 patient. H&E (200×) stain section demonstrates the typical wavy stroma with the S-100 positive elongated nuclei, with occasional intratumoral fascicles. Fibroblasts, mast cells, and pericytes are also present. (C) Paraganglioma: H&E (400x) section demonstrating the round nucleated cells with the typical lobular architecture. Neurosecretory granules are abundant and seen on electron microscopy. (D) Intradural extramedullary meningioma (T1 and gadolinium) in the lower thoracic spine with deviation of the thecal sac. H&E (200×) section demonstrating the whorls of a typical meningioma. (E) Hemangioblastoma of the lower thoracic-conus region in a non-von-Hippel Lindau patient. Sagittal MRI (T1 and T2) demonstrates the flow voids, mural nodules, and serpiginous vessels (seen on the selective spinal angiogram below). H&E (630×) demonstrates the highly vascular nature of the lesion, lipid-laden cells. These tumors may be totally intramedullary, often associated with a cyst, or have an extramedullary component arising from the nerve root.
Several tumors are noted to be predominantly paraspinal in location, with varying degrees of intraspinal extension. These lesions have been classified by Mc-Cormick3 into four types: I, dumbbell tumors with significant intraspinal and anterior paraspinal components; II, tumors confined to the anterior paraspinal region; III, anterior spinal tumors with minor intraspinal or foraminal extension; and IV, tumors with significant vertebral epidural and paraspinal involvement. There are a variety of pathologic lesions seen in the paraspinal region, with the classification determined by radiologic imaging of tumor extension, as it has significant implications toward surgical planning for tumor removal and spinal stabilization.
Neoplasms arising primarily from the spinal column include vertebral hemangiomas, which have increased T1 and T2 signals on MRI. The hyperintense T1 signal is secondary to the fatty component, with the increased T2 signal secondary to the increased cellular component of these tumors. These hemangiomas are often indolent and recognized by their characteristic “thickened trabeculated” appearance on CT and plain films. Osteoid osteomas are characterized by a radiolucent area with a central nidus and surrounding sclerosis, whereas osteoblastomas are larger tumors with less abundant sclerosis. Both of these tumors have intense enhancement after contrast or radionucleotide uptake on bone scans, with the central nidus being relatively “cold.” Other primary bony tumors include aneurysmal bone cysts and giant cell tumors, both of which are characterized by cystic lesions, with malignant osteosarcomas demonstrating a combination of lytic and blastic destructive alterations. These primary bony neoplasms may present with progressive scoliosis and pain secondary to pathologic fractures, with occasional accompanying neurologic deficits.
The most common CNS derivative neoplasms in the conus and cauda region are ependymomas of the filum terminale and exophytic astrocytomas and other gliomas from the conus. Ependymomas, of which the myxopapillary variant is most common in this region (Fig. 22-2), enhance brightly and are hypointense on T1- and hyperintense on T2-weighted MRI. Astrocytomas and gliomas are rarer and tend to be hypo- to isointense on T1 and hyperintense on T2-weighted MRI, with variable gadolinium enhancement.
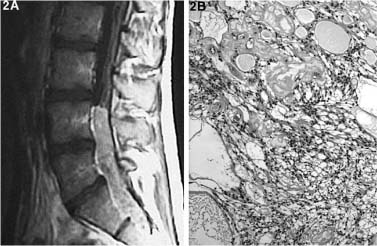
FIGURE 22-2 Myxopapillary ependymoma of the cauda equina, arising from the filum terminale (T1 and gadolinium). H&E (100×) section demonstrates the loose stroma, with the cells often organized in a papillary form around areas of mucin that can have a central blood vessel.
Surgical Pathology (Table 22-2)
Nerve sheath tumors (schwannomas and neurofibromas) in the conus and cauda region are pathologically similar to their more common origin from peripheral nerves (Fig. 22-1A,B) and are discussed in that section below. Nerve sheath tumors in the paraspinal region with both extradural and intradural extension pose special management issues compared to those in the extremity, but the objectives of tumor removal and sparing of passerby and functional fascicles are similar. About 15% of these tumors are purely extradural.4 In patients with NF1, multiple paraspinal neurofibromas may be present and require long-term clinical and radiologic follow-up by MRI. Although neurofibromas demonstrate intraneural growth, significant debulking can be undertaken when these paraspinal neurofibromas become potentially symptomatic by compression of intracanalicular neural elements. Rapid growth accompanied by increased symptoms of these multiple paraspinal neurofibromas also raise the possibility of malignant transformation to a MPNST (Fig. 22-3), a diagnosis with significant implication requiring accurate diagnosis, radical surgery to obtain tumor free margins, and adjuvant therapy including radiation and potentially chemotherapy.
Meningiomas are mainly localized to the thoracic region (Fig. 22-1D), with a large female preponderance, and are relatively rare below the level of the conus medullaris.5 They usually originate in the ventrolateral aspect of the spinal canal, and although they often have an intimate relationship to the nerve roots, they can usually be separated from them. Enlargement of the foramen, a feature associated with tumors originating from the nerve sheath, is usually not present, though some spinal meningiomas can have a significant extradural component and deform the foramen. About 15% of spinal meningiomas are extradural.6
Paragangliomas of the cauda equina are composed of clusters of large uniform polygonal cells with pale eosinophilic granular cytoplasm, which are sharply circumscribed by a fibrovascular stroma (Fig. 22-1C). There may be pseudopapillary structures, similar to ependymomas; however, glial fibrillary acidic protein (GFAP) stain is negative.7 In about half of paragangliomas, mature neurons are scattered among the tumor cells, with these tumors being labeled as “ganglionic variety” of paragangliomas. Tumor cells stain positive for neuronal differentiating markers such as neuron-specific enolase (NSE) or protein gene product 9.5 (PGP9.5), with synaptophysin positivity confirming the presence of neurosecretory granules being more specific for paragangliomas.8,9 Histologically, it is very difficult to differentiate between the benign and malignant paragangliomas, the latter demonstrating not only rapid local growth but also metastasis. Expression of certain neuropeptides like Leuenkephalin, Metenkephalin, somatostatin, and pancreatic polypeptide have been suggested to correlate to the biologic behavior of paragangliomas,10 though this has not been conclusively demonstrated.
Hemangioblastomas (Fig. 22-1E) can occur as a part of the predisposition for von Hippel-Lindau syndrome or as a solitary tumor.11,12 These tumors are rare in the cauda equina region, where they usually arise from the conus medullaris, with an occasional origin reported from the filum terminale.13–15 Characteristic of all hemangioblastomas, they are highly vascular intradural spinal tumors that can often have an associated syrinx. In familial cases the tumors tend to be multiple. With rare exceptions they are benign tumors. Sometimes the tumor may spread along the subarachnoid space after an operative procedure but remains benign. Distant metastasis has been reported. Although they do not have a true capsule, they are well circumscribed. The tumors can be solid or cystic. Histologically the tumor is composed of three groups of cells: endothelial cells, pericytes, and stromal cells. The cardinal feature of the tumor is the presence of numerous anastomosing capillary channels that form a plexiform pattern and are lined by a single layer of plump endothelial cells. Although several researches have been conducted in search of the origin of the tumor cells, the final word has not come as yet.
Ependymomas (Fig. 22-2) are the second most common tumor of the conus and cauda equina, after schwannomas and neurofibromas, and the most common one originating from a CNS cellular element. In the context of the predisposition syndrome NF2, where spinal manifestations are common in addition to the pathognomonic bilateral vestibular schwannomas, intramedullary spinal lesions are most often ependymomas. Although most of the spinal ependymomas arise from the central canal in the cervical or cervicothoracic region, ~40% of them arise from the vestigial central canal of the filum terminale.16,17 These ependymomas arising in the conus and cauda have a characteristic myxopapillary pathologic feature, which at surgery may be difficult to differentiate from a nerve sheath tumor arising from a nerve root. Ependymomas arising predominantly from the conus can extend rostrally in the spinal cord with an associated tumor-related syrinx, along with exophytic caudal extension into the cauda equina. Myxopapillary ependymomas are rarely malignant biologically but are known to seed the craniospinal axis with occasional systemic metastasis. Pathologically these distant metastases are usually not malignant in nature and appear similar to the primary myxopapillary ependymoma, suggesting the mechanism of distant metastasis to be secondary to local CSF and venous invasion rather than malignant transformation.18
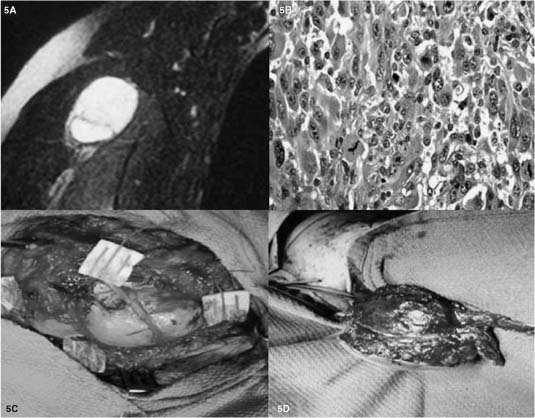
FIGURE 22-3 Malignant peripheral nerve sheath tumor (MPNST) arising from an infraclavicular brachial plexus plexiform neurofibroma in an NF1 patient. (A) MRI (T1 and gadolinium), with no pathognomonic features to differentiate from benign tumors such as schwannomas or neurofibromas. (B) H&E (400×) demonstrates hypercellular, nuclear pleomorphism, and high mitotic activity (>10/HPF). (C) Intraoperative picture demonstrating the principle of quadrant biopsy and subsequent closure to first pathologically verify the MPNST. (D) Second intraoperative picture after pathologic verification (patient first underwent preoperative radiation) demonstrates resection of the MPNST and the overlying and adjacent soft tissues, including the proximal and distal portion of the nerve of origin, in an attempt to obtain tumor-free margins and local control.
Astrocytomas and oligodendrogliomas originate from the conus medullaris, are often associated with a rostral syrinx, with only a few case reports of predominant extramedullary exophytic caudal growth.19 Like their counterparts elsewhere in the CNS, astrocytomas and oligodendrogliomas of the conus have an infiltrative and invasive growth pattern, usually without a discrete tumor-cord interface. The majority of these gliomas are of low grade (grade I or II), with occasional calcifications at presentation; however, like their intracranial counterparts, these low-grade gliomas do progress biologically, especially in adults, with about one fifth presenting with malignant grades III or IV lesions.
Epidermoid and dermoid cysts are dysembryonic inclusion cysts, with accumulation of cyst contents secreted by the outer membrane.20,21 Epidermoid and dermoid cysts are usually slow growing, avascular, and encapsulated, enabling relatively easy internal radical debulking; however, the capsular reaction with surrounding neural tissue may be intense and limiting, which with their extreme slow growth rate often leads to subtotal resection. Epidermoid tumors of the cauda equina are usually associated with a preceding history of lumbar punctures, theorized to carry cutaneous tissue into the spinal canal, which acts as the nidus. In dermoid cysts, the surgeon must be vigilant in searching for a communication with the surface, which must also be resected to prevent recurrent meningitis and other complications. When the dermoid cyst is associated with tethering of the filum terminale, resulting in neurologic dysfunction, especially during pubertal growth spurt, untethering becomes the primary surgical objective. Several other developmental-related benign tumors of vestigial elements may be encountered in the conus and cauda region, which are described in detail in Chapter 8. These include teratomas, lipomas, and spinal hamartomas, the latter representing a tumor of well-differentiated mature elements in an abnormal location.22
Several primary benign and malignant tumors of primary osseous origin occur in the thoracolumbar region. These lesions can cause symptoms by either direct extradural extension compressing the conus, and/or the exiting roots through the neural foramina, or secondary to pathologic fractures of the vertebral column. These are discussed in greater detail in Chapter 23 and include benign lesions such as the following: (1) Hemangiomas are the most common benign bony tumor of the spine. They are of vascular origin and are usually asymptomatic and are incidental radiographic findings, which can occasionally be associated with pathological collapse of the vertebral body.22 (2) Aneurysmal bone cysts are common indolent lesions that occur predominantly in the posterior elements in young adults,23 though they can also involve the vertebral body.24 Microscopically they are composed of mainly fluid-filled spaces separated by fibrous septa. (3) Giant cell tumors more commonly occur in the vertebral body and are composed of multiple giant cells of uncertain origin with a fibrous stroma. Most of these tumors are benign, though they can recur and rarely have been documented to metastasize.25,26 (4) Eosinophilic granulomas are benign lesions of children and teenagers, characterized by histiocytes and eosinophils. Classically, they present with a symmetric flattened vertebral body (vertebra plana) or a lytic lesion of the body without collapse. (5) Osteoid osteomas tend to occur mainly in the posterior elements of the lumbar spine (lamina and pedicle) of children and young adults.27 (6) Osteoblastomas also commonly occur in the posterior elements of young adults, though with equal predilection for the entire spinal axis.22,27 This lesion as well as osteoid osteomas are benign and pathologically similar, with osteoblastomas being greater than 1.5 cm. (7) Osteochondromas are the most common of the primary osseous tumors and tend to involve mainly the spinous process. Macroscopically, they are cartilagecapped bony prominences, which are usually benign in solitary osteochondromas but can degenerate into chondrosarcomas when they are part of a cancer predisposition syndrome with multiple lesions present.
The most common malignant tumors of the spine are metastasis, as discussed below. Primary malignant osseous spinal lesions include the following: (1) Osteosarcomas are extremely rare, as most are a metastasis from their more common origin in long bones or are radiation-induced tumors or arise from preexisting Paget’s disease.22,28,29 (2) Chondrosarcomas can occur primarily or secondary to malignant degeneration of a preexisting osteochondroma, with a biologic behavior that is unpredictably variable and not easily predicted from the histologic appearance.22 (3) Ewing’s sarcoma is a relatively common malignant tumor of children and young adults, which rarely occurs primarily in the spine, though metastases to the spine are not uncommon.30 (4) Multiple myeloma is the most common malignant neoplasm of bone in older adults. (5) Chordomas are rare primary malignant tumors arising from notochord elements. The majority arises in the sacrococcygeal region, with the occipital-clival junction being second and the thoracolumbar spine rarely involved. Chordomas are lobulated gray partially translucent cystic or solid masses with a distinct lobular architecture formed by the pathognomonic “physaliphorous” cells with ample vacuolar cytoplasm and signet ring cells.
Metastatic tumors are the most common malignant tumors of the spine, involving the spinal column or extradural space. Metastases are usually limited to the extradural space, as the dura acts as a natural barrier against spread to the subarachnoid or subdural space. Intradural leptomeningeal metastases are rare and usually arise from adenocarcinomas and leukemias, though subarachnoid seeding from intracranial primary CNS tumors such as those in the primitive neuroectodermal tumor (PNET) family (medulloblastomas, pinealoblastomas, neuroblastomas) or, rarely, astrocytomas can also occur. Pure intradural extramedullary metastases are also extremely rare and are estimated to be about 1 to 4% of all spinal metastasis.31,32 In adults, the usual sources of extradural metastases are lung, breast, colon, prostate, and lymphoma. These reflect the normal population cancer burden, with the spinal metastasis representing the first manifestation of the malignancy ~10% of the time.33 Metastasis to the spinal axis usually occurs through arterial channels to the bone marrow of the body or posterior elements and then into the epidural space via venous channels. In prostate and colonic tumors, retrograde metastasis via the Batson’s venous plexus is another common mode of seeding.34 Most of tumors are localized to one or two adjacent vertebral segments, with about 17 to 30% of patients showing evidence of cord or cauda equina compression at two or more noncontiguous sites.35,36 Certain tumors such as lymphomas often can have extensive extradural involvement without any bony alterations, a radiologic clue of the nature of the primary tumor.37,38
Treatment
The two main principles for treatment of neoplasms in the region of the conus and cauda equina are (1) to achieve decompression of neural elements and (2) to achieve a stable spine, which may have been compromised by the tumor or the required decompression during surgery. The patient must be aware of both of these objectives and the anticipated risks to motor, sensory, and bladder and bowel function. To reach these objectives, the surgical team, which often may be multidisciplinary, should be versed in posterior, lateral, and anterior approaches to the thoracolumbar region, along with the various modalities of stabilization that are available. The plain films and MRI often need to be complemented with CT scanning and reconstruction to better define the preoperative bony anatomy and to optimize the selection of the available approaches and the requirement for stabilization. Occasionally, there is a need for preoperative spinal angiography to better delineate the vascular supply and undertake embolization to minimize blood loss during tumor removal.
The approach to be utilized usually dictates the choice of patient positioning, though sometimes the decompression and supplemental stabilization through a second approach is best staged and requires patient repositioning. For most intradural and epidural lesions in the region of the conus or cauda equina a posterior or posterolateral approach suffices, with the patient in the prone position. In selected cases, where the extension of the tumor into the abdomen or pelvis requires a more anterolateral retroperitoneal approach, a lateral position can be utilized. Care must be taken to minimize abdominal compression and all pressure points are padded to minimize venous congestion and guard against pressure palsy. Foley catheterization; adequate arterial monitoring; and intravascular access, as anticipated by the length of the procedure, expected blood loss, and medical status of the patient are required. Perioperative antibiotics and steroids are usually used. It is crucial to have intraoperative electrophysiologic monitoring with reproducible baselines prior to commencing, with the surgeon conveying this requirement to the anesthetist. Somatosensory-evoked potentials (SSEP) from both the upper (to serve as an internal positive control) and lower limbs are used. In lesions of the cauda and conus, direct audible electromyography (EMG) of selected muscles conforming to the vulnerable nerve roots and monitoring of the external bladder sphincter are often valuable.
The primary goal is for neural decompression, the degree of which is dictated by the anticipated biology of the neoplasm as determined by the quick section and the plane of resection between the tumor and neural elements that one is trying to preserve. Adequate exposure through the various approaches above and below the tumor is vital to successful and safe removal of the tumor, recognizing but not limited by the need for stabilization. General details of undertaking the standard posterior, posterolateral, and anterolateral approaches are covered in Chapters 30, 33, 35, and 38. For primary intradural lesions, a midline durotomy preserving the arachnoid, which is subsequently opened and tacked to the dura, with diligent hemostasis so as not to contaminate the operative field, is desirable. The rostral and caudal poles of the tumor, along with the intermingled neural structures, which may be deviated in variable directions depending on the origin and nature of the tumor, are defined and marked with patties under microscopy as best as possible. This is followed by electrophysiologic definition of the visible neural structures to confirm the location of the conus, the motor and sensory rootlets, and the filum prior to tumor decompression whenever possible. In many situations, partial tumor debulking in an electrophysiologically safe region is first required to define the obstructed and deviated neural elements. A quick section done relatively early during tumor removal is recommended, as the biologic nature of the tumor is often the best guide to the extensiveness and goals of surgery. Microscopic observation as to the origin, the nature of the tumorneural structure interface, vascularity, and other features are also important determinants of the surgical goals and predictive of the pathology. For example, a fusiform intraneural origin from the cauda equina is highly suggestive of a neurofibroma as opposed to a more discrete lobular origin with extraneural growth of a schwannoma. A lesion arising from the conus or filum with a large exophytic component and a relatively distinct tumor-conus interface is suggestive of a myxopapillary ependymoma,39 as opposed to a conus lesion with rostral infiltrative extension typical of astrocytomas or other invasive gliomas. A highly vascular lesion with serpiginous vessels, a mural nodule, and associated syrinx are all suggestive of hemangioblastomas, a diagnosis that is usually made reliably on preoperative MRI scanning.
The general principles of tumor removal include preservation of the functioning neural elements, control of the main vascular supply as early as possible, and preservation of the arachnoid plane during dissection whenever possible. Piecemeal tumor removal is usually undertaken with intracapsular debulking using the Cavitron, with traction on the tumor rather than on the neural tissue. In tumors with significant extraspinal extension, after adequate intraspinal neural decompression has been first undertaken, additional posterolateral or anterolateral extension may be required. Type I paraspinal dumbbell tumors as per McCormick’s classification3 can usually be removed by posterolateral extension after intracanalicular decompression has been achieved. In small lesions with limited destruction of the pedicle, stabilization may not be required, whereas posterior stabilization involving the adjacent levels may be indicated with larger tumors. Pure type II paraspinal anterior-located tumors usually require an extraspinal anterolateral approach, whereas type III anterior paraspinal lesions with minor intraspinal and foraminal involvement may require a staged posterior and anterolateral approach often undertaken at the same setting, with added posterior stabilization sometimes indicated. In type IV paraspinal lesions with significant vertebral and epidural involvement, a combined anterior and posterior approach or a lateral approach is most likely indicated with combined anterior and/or posterior stabilization. As stated earlier, surgical decisions and objectives are a composite based on a combination of patient characteristics, biology of the tumor, and intraoperative microscopic and electrophysiologic observations. Although the ideal goal may be total tumor removal, it may not always be the most appropriate based on the information accumulated from the above sources, with either postoperative follow-up or adjuvant radiation and/or chemotherapy required after partial removal.
 Peripheral Nerve Tumors
Peripheral Nerve Tumors
Peripheral nerve tumors are subdivided into benign and malignant and also primary or the rare secondary tumors that abut or sometimes directly infiltrate the nerve (Table 22-3). The cancer predisposition syndrome, neurofibromatosis type 1 (NF1), is commonly associated with peripheral nerve tumors, and hence the clinical and molecular diagnosis of this syndrome is reviewed (Table 22-4). A MPNST, in the context of NF1 or sporadic patients, is a rare tumor that needs to be managed in specialized centers, as outlined below.
Diagnosis and Management
History and Physical Examination
When a peripheral mass is associated with sensory or motor symptoms supplied by a known peripheral nerve, the suspicion of a peripheral nerve tumor is readily apparent; however, most peripheral nerve tumors present without neurologic symptoms due to their slow growth rate or origin from a superficial small sensory branch, and in these cases a wide differential diagnosis of peripheral masses including lymph nodes and benign or malignant soft tissue tumors has to be entertained. Several features of the clinical examination of an extremity mass are suggestive of a peripheral nerve origin: (1) Peripheral nerve tumors are mobile perpendicularly but not along the longitudinal axis of a known peripheral nerve. (2) Palpation of a peripheral nerve tumor may elicit sensory stimuli radiating along the distribution of the nerve of origin. (3) A mass in the presence of a patient with a genetic predisposition such as NF most likely represents a peripheral nerve tumor.
Features of the mass such as its growth rate, association with pain, fluctuance, overlying skin temperature and color; the patient’s general health including immune status; and preexisting malignancy are also helpful in the differential diagnosis of an extremity mass. A family history of extremity tumors, whether or not in the context of NF, should also be sought, along with the systemic examination of the patient looking for the clinical features of NF1, as discussed later and outlined in Table 22-4. A thorough history and physical examination not only are the major sources of information for the diagnosis of a peripheral nerve tumor but also are vital in the decision-making process for their proper management. Due to the rarity of peripheral nerve tumors and a wide differential diagnosis, these patients are often initially referred to other surgical disciplines and subsequently sent to a peripheral nerve unit after biopsy. It is recommended that if a clinical suspicion of a peripheral nerve tumor, as per the discussion above, is entertained, or if the initial biopsy reveals a peripheral nerve tumor, the patient should be referred to a multidisciplinary peripheral nerve unit for optimal definitive management.
| Benign neural sheath tumors | Schwannomas |
| Neurofibromas (solitary versus NF1; dermal, plexiform) | |
| Pericytomas | |
| Neurothekomas | |
| Malignant tumor of nerve sheath | Malignant nerve sheath tumors (MPNST) |
| Tumors of nonneural sheath origin | Desmoid tumors, myoblastomas/granular cell tumors |
| Lipomas, lymphangiomas, meningiomas | |
| Ganglion cysts, primitive neuroectodermal tumors | |
| Neuroblastomas, ganglioneuromas, ganglioneuroblastomas | |
| Chemodectomas, pheochromocytomas | |
| Tumors of vascular origin | Hemangiomas, venous aneurysms or angiomas |
| Hemangioblastomas, hemangiopericytomas | |
| Secondary malignant nonneural tumors | Soft tissue sarcomas, Pancoast tumors, lymph nodes |
| NF1: Two or more of the following |
| Six or more café-au-lait macules of over 5 mm in greatest diameter in prepubertal individuals, and over 15 mm in greatest diameter in postpubertal individuals |
| Two or more neurofibromas of any type or one plexiform neurofibroma |
| Axillary or inguinal freckling |
| Optic glioma |
| Two or more Lisch nodules (iris hamartomas of melanotic origin) |
| A distinctive osseous lesion such as sphenoid dysplasia or thinning of the long bone cortex with or without pseudarthrosis |
| A first-degree relative (parent, sibling, or offspring) with NF1 by the above criteria |
Preoperative Tests
Nerve conduction and EMG evaluation are routinely not undertaken in the management of peripheral nerve tumors. The importance of the history and physical examination is further emphasized due to the limitation of radiologic studies in differentiating between a peripheral nerve tumor and other soft tissue extremity masses. In addition, CT and MRI scans cannot distinguish between the various subtypes of peripheral nerve tumors or determine whether a lesion is benign or malignant.40–43 MRI scans are the most useful and sensitive technique, often but not always revealing the nerve of origin. It is especially useful in determining the relationship of the mass to adjacent anatomic structures, which are of relevance. Occasionally, adjacent bony remodeling secondary to the slow progressive growth of the tumor, such as enlargement of neural foramina, can be better visualized on plain x-rays or CT scans, but MRI scanning is the investigation of choice.
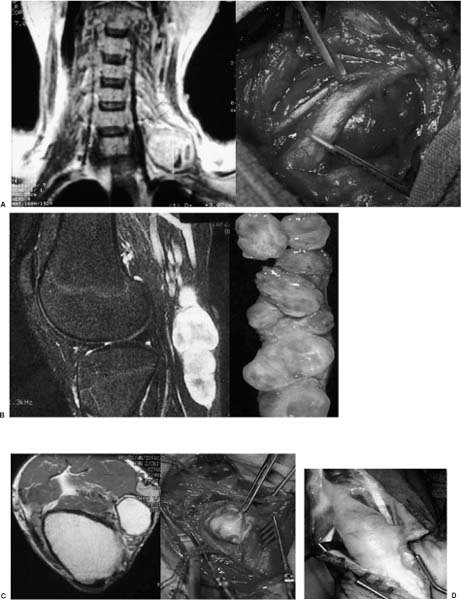
Although not pathognomonic, some features on the MRI scans may help in the preoperative differential diagnosis of peripheral nerve tumors. Schwannomas usually have a low T1 and high T2 MRI signal, with marked homogeneous contrast enhancement (Fig. 22-4A). Occasionally, the nerve of origin or exit can be visualized, sometimes accompanied by the displaced fascicles around the tumor circumference. In contrast to neurofibromas, schwannomas tend to have a discrete boundary with an elliptical or spherical shape. As noted, these radiologic features have to be put in the context of the history and physical findings. The presence of NF1 makes the diagnosis of a peripheral nerve tumor being a neurofibroma more likely. In addition, a fusiform or multinodular shape with lack of visualization of a distinct nerve of origin or exit, and passerby fascicles such as those found in typical schwannomas, are suggestive but not pathognomonic of a neurofibroma (Fig. 22-4B). The rare ganglion cysts (Fig. 22-4C) and lipomas (Fig. 22-4D) have characteristic MRI signal changes, with association to a nearby joint capsule for ganglion cysts, making the preoperative diagnosis quite apparent.
Whether a peripheral nerve tumor is benign or represents an MPNST (Fig. 22-3) cannot be definitely determined on MRI or CT scan characteristics.40–43 A positive gallium scan was reported in one small series to be specific for MPNST, but follow-up studies involving larger numbers are not available.41 Regions of nonhomogeneous enhancement on CT or MRI, suggestive of intratumoral necrosis or hemorrhage, indicate a malignant and aggressive MPNST but are also found in benign schwannomas and neurofibromas. Recent unpublished reports from the United Kingdom suggest that positron emission tomography (PET) scans may be efficacious in differentiating benign neurofibromas from MPNSTs of either low or high grade. The suspicion of a malignant tumor originating from a peripheral nerve or secondary involvement of a nerve from an underlying primary neoplasm (e.g., Pancoast tumor) comes from the history and general physical examination. Rapid growth clinically or on follow-up MRI scans, increasing neurologic symptoms, or preexisting NF1 with a plexiform neurofibroma should make one think of MPNST and follow the management strategy as outlined below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree