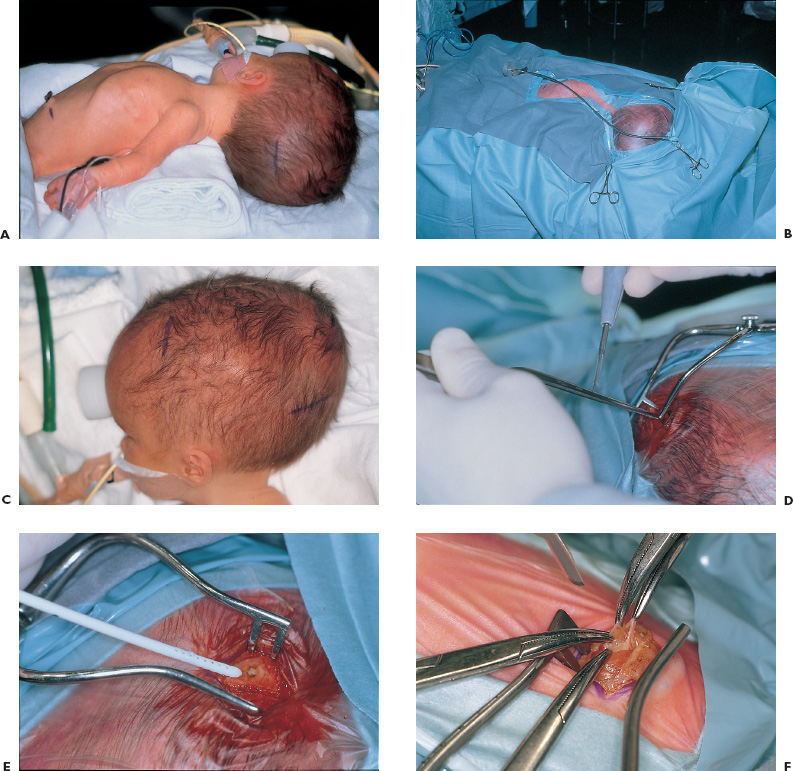
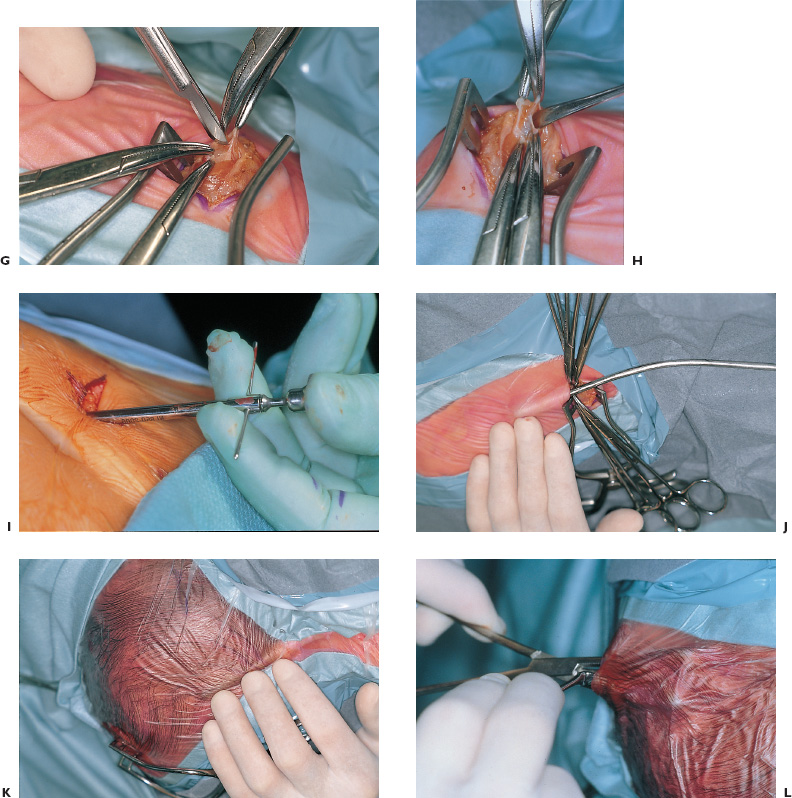
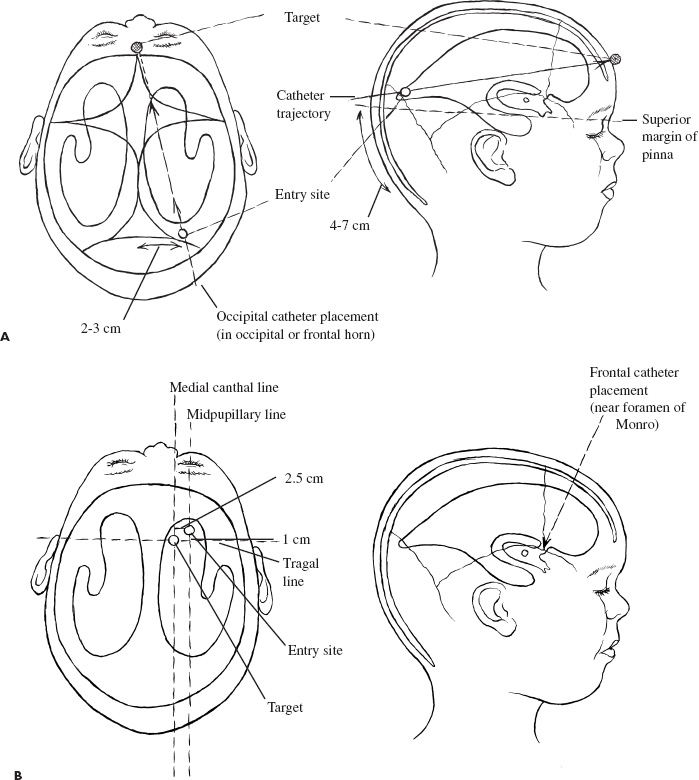
Congenital and Developmental Disorders Hydrocephalus is the most frequent neurosurgical problem encountered in the pediatric age group. With an incidence of 1 in 2000 births, it occurs in nearly one third of all congenital malformations of the nervous system. The treatment of hydrocephalus was revolutionized following the introduction of valved shunt systems by Nulsen and Spitz in 1952 and the subsequent development of silicone shunt systems by Holter and Pudenz. Cerebrospinal fluid (CSF) shunting procedures constitute approximately half of most pediatric neurosurgical practices, with roughly half of these procedures performed for revision of an existing shunt. Clearly, despite recent advances in shunt design, they continue to present a technical challenge for the neurosurgeon treating children with hydrocephalus. Infants and children who present with ventriculomegaly and clinical evidence of elevated intracranial pressure clearly require a CSF diversionary procedure. Whereas conventional CSF shunting procedures have long been the main treatment option in these children, dissatisfaction with their long-term outcome and significant failure rates has resulted in a resurgence of interest in the earliest form hydrocephalus treatment: ventriculostomy. Patient groups exhibiting appropriately high success rates following third ventriculostomy are still being defined, however, and probably represent a minority of patients with hydrocephalus. CSF shunting procedures are likely to remain the treatment of choice for most children with symptomatic ventriculomegaly. The decision to treat ventriculomegaly becomes more difficult in the patient whose symptoms are minimal, absent, or difficult to assess because of the patient’s age. Radiographic progression of ventriculomegaly over time, even if asymptomatic, is generally considered an indication to treat, unless it is secondary to cortical atrophy associated with anoxia, syndromic malformations, or degenerative/metabolic disease. Less agreement exists, however, about the treatment of asymptomatic stable ventricular enlargement. The inconsistent use of the terms arrested and compensated hydrocephalus adds to this controversy. Patients with stable ventricular enlargement resulting from age-related CSF absorption abnormalities (e.g., benign extraaxial fluid collections of infancy) or venous occlusive disease (e.g., pseudotumor cerebri, achondroplasia) generally are not considered for treatment unless radiographic or clinical progression is clearly documented. Children with stable clinical and radiographic findings over the age of 5 years can be monitored closely for evidence of subtle abnormalities in intellectual development. It is important to weigh the known long-term risks of shunt complications against the largely unknown impact of asymptomatic hydrocephalus in this group of patients. Younger children, particularly those under the age of 3 years, cannot be evaluated by standard measures of intellectual development. Many authors recommend treatment of asymptomatic patients with moderate to severe ventriculomegaly in this age group to protect against the impact of ventriculomegaly on future intellectual performance. Unfortunately, imaging studies and invasive testing, including infusion tests, radionuclide studies, Doppler screening, intracranial pressure monitoring, and magnetic resonance (MR) spectroscopy, to date have been unable to predict reliably which of these children may be at risk for intellectual decline. The diagnosis of hydrocephalus is based on clinical and radiological features. Infants most commonly present with symptoms of irritability, delayed development, vomiting, and headache and on examination have increasing head circumference and a bulging fontanel. MR imaging has the best diagnostic utility in terms of establishing the anatomy and likely pathology of potential obstructive lesions. Computed tomography (CT) is usually sufficient for follow-up of previously diagnosed patients with existing shunt systems and preliminary or urgent evaluations of undiagnosed patients. Ultrasound is more practical in critically ill, premature infants with intraventricular hemorrhage or in infants with myelomeningocele the etiology of which is not in doubt. Moderate to severe ventriculomegaly usually can be visualized easily using any of these techniques. In patients with milder ventricular enlargement, evidence of transependymal flow of CSF usually suggests that the process is more acute. Other signs of progressive hydrocephalus—such as enlargement of the temporal horns, dilatation of the third ventricle, and effacement of the sulci—are less specific. If doubt exists, careful observation with serial images, rather than subjecting the patient to the known risks of shunt failure, is prudent. An appreciation of the epidemiology and presentation of shunt failure resulting from mechanical or infectious complications is essential in determining appropriate shunt hardware and insertion techniques. The most common time for a shunt failure is in the first 6 months following insertion. Overall 1-year failure rates approach 40%. Most centers report infection rates on the order of 5 to 10%. These infections usually present within 2 months of shunt insertion, indicating that most infections occur during shunt surgery itself. Although the mechanism of shunt contamination seems relatively straightforward, numerous studies instituting procedures aimed at risk reduction have failed to demonstrate consistently that these interventions led to lower infection rates in some centers. A detailed history, including all the previous shunt surgeries, is mandatory. Previous notes from the operating room (OR), from whatever hospitals the surgery was done, should be sought. The relevant imaging, which should be related to the patient’s clinical status at the time they were obtained, also should be carefully studied. This entire process often requires creating a spreadsheet or log to keep track of the multiple interventions. All previous culture reports also should be sought, looking for an unrecognized or partially treated infection. Current imaging should be complete, including plain radiographs of the shunt equipment. Examination of the site of the shunt equipment implantation may provide confirmatory evidence of shunt dysfunction. Although pumping of the shunt reservoir is a time-honored technique, in fact, it is often misleading. A patient in whom a reservoir fills slowly may simply have small ventricles; however, shunts whose reservoirs remain umbilicated for prolonged periods, or even permanently often are blocked proximally. A reservoir that is quite difficult to depress or that refills apparently instantaneously frequently indicates a distal obstruction. Fluid collecting around the shunt, particularly if it firmly distends the skin, is progressive, and tracks along the distal catheter, is often a sign of shunt occlusion. When shunts fracture, CSF often continues to track along the fibrous sheath. In this scenario, often a small amount of fluid and a space where the shunt has come apart can be felt. It may be difficult, however, to distinguish an empty sheath from the sheath containing shunt tubing, particularly if the shunt has been implanted for some years or the tract is calcified or distended with fluid under high pressure. A fluid thrill sometimes can be felt at the site of a distal catheter disruption with pumping of the proximal reservoir. Infection must be ruled out in all patients presenting with early shunt malfunction (within 2–6 months of insertion) and in patients with clinical features suggesting infection (e.g., meningismus, fever, elevated white cell counts, abdominal pain). Exclusion of infection is achieved most conclusively by sampling the CSF, usually from the shunt reservoir itself. CSF samples from patients with isolated distal shunt infections often are sterile, however, making abdominal ultrasound or CT necessary to rule out intraabdominal abscesses or “pseudocysts.” These studies are especially important for patients presenting with predominantly abdominal symptomatology. All children with suspected shunt infection should have a thorough history and physical examination to rule out other common febrile illnesses, such as otitis media and viral gastroenteritis, which can be indistinguishable from a shunt infection. Likewise, urinary tract infections always should be considered in myelomeningocele patients. No data are available to determine which particular shunt should be recommended; in fact, a recent randomized trial on CSF shunt design comparing a standard differential pressure valve to both siphon-limiting (Delta valve, Medtronic PS Medical, Goletta, CA) and flow-limiting valves (Orbis-Sigmam NMT, Boston, MA) failed to show any difference in terms of overall shunt failure. Important considerations must be taken into account, however, when assessing the individual patient: age, weight, skin thickness, head size, size of the ventricles, pathogenesis of hydrocephalus, acuteness of the illness, presence of internal lines or gastrostomy, tracheotomy openings, status of the distal drainage site, and plans for further surgery. For example, a premature infant with thin skin stretched further by a rapidly expanded head cannot accommodate adult-sized equipment without the risk of skin erosion. The presence of intraventricular hemorrhage in this patient group also may increase the risk of early obstruction following implantation of a valve with a narrow flow-limiting orifice. Conversely, in patients with large ventricles and large skulls with fused sutures, placing a flow-limiting or siphon-reducing device may decrease the risk of subdural hemorrhage. Ventricular configuration and asymmetry also may dictate the choice of insertion site. In addition to patient characteristics present at the time of initial shunt insertion, it is important to consider future treatments that may affect shunt function. For example, endoscopic fenestration of a loculated ventricular system when the shunt is inserted may be indicated to decrease the number and complexity of future shunt systems. Patients who have elaborate shunt configurations with several connections, or multiple separate shunts, suffer higher rates of shunt failure than those who have simple shunts. Metallic shunt components or magnetic programmable valves should be avoided in patients expected to require multiple MR imaging studies to avoid degradation of the images and unnecessary reprogramming of the valve. Finally, if a patient is scheduled to have further intraabdominal surgery, for example, colostomy closure or bladder reconstruction/augmentation, the preferred distal drainage site may be altered. For most routine cases, the surgeon probably should choose familiar shunt equipment and use the same system consistently. Current evidence does not support the superiority of any particular valve design, and so the individual surgeon’s technique and ability to evaluate outcomes are likely enhanced by this approach. In this situation, we prefer a two-piece system with a nonflanged ventricular catheter, connected to a flat-bottomed valve with a reservoir, and integrated open-ended distal tubing. Shunt surgery is often regarded with some disdain by staff and trainee neurosurgeons alike; it has the highest failure rate of any neurosurgical procedure, and nothing is less forgiving of any technical errors than a shunt operation. It is well known that shunts frequently fail as a result of tissue occluding the proximal or distal catheter. Avoidable complications, such as intraparenchymal ventricular catheters, extraperitoneal distal catheters, and spontaneously disconnected or migrated shunts, have occurred on virtually every neurosurgical service. Shunt surgery should command great respect, require meticulous attention to detail, and be carried out in a skilled and expeditious fashion. Body wash and shampoo the night before and again just prior to surgery using an antiseptic solution (e.g., chlorhexidine) are recommended. A number of metanalyses have shown that prophylactic antibiotics are effective, and they are strongly recommended. Likewise, all shunt equipment is soaked in antibiotic-containing solution prior to insertion. For patients in whom an abdominal trochar is being used, the bladder should be emptied either by a Crede maneuver or by urinary catheter. Hair removal has never been shown to decrease the risk of infection; however, the hair can be clipped (not shaved) in the OR if so desired to assist with skin closure and application of postoperative dressing. The patient should be positioned so there is a flat plane between the upper and lower incision sites, facilitating subcutaneous passage. For an occipital burr hole, this means rotating the head to the opposite side, and extending the neck, usually with a rolled towel (Figs. 1–1A and B). The site of the burr hole and abdominal incisions should be selected and marked before draping and before the surface landmarks are obscured. Placement of a surface marker such as an electrocardiographic (ECG) electrode that can be palpated through the drapes may assist in placement of the ventricular catheter (Figs. 1–1C). FIGURE 1–1. A: Patient positioning and marking of incisions for ventriculoperitoneal shunt. B: Draped patient with passer, illustrating planned subcutaneous path of the shunt. C: Proposed incisions for occipital or frontal shunt placement, with plastic syringe cap used for landmark. D: Monopolar cautery applied to bayoneted forceps. E: Result of (D), a small dural opening for the ventricular catheter. Sequential steps in accessing the peritoneal cavity; F: Elevation of peritoneal layer. G: Sharp opening. H: Probing peritoneal cavity with blunt instrument to confirm entry. I: Alternative peritoneal access using an abdominal trochar. J: Insertion of tunneling instrument initially angled posteriorly to ease passage over the chest. K: Passer rotated to angle anteriorly in the neck to pass over occiput. L: Blunt dissection with a hemostat to create a subcutaneous “pocket” for the valve. The issue of frontal versus occipital burr hole has never been resolved. We generally prefer an occipital shunt placement if the occipital horns are sufficiently enlarged because it allows insertion of the entire shunt system without an intervening neck incision. Usually, occipital burr holes are placed on the flat part of the occiput 3 to 4 cm from the midline along the course of the lambdoid suture (Fig. 1–2A). Patients with a Dandy-Walker malformation or large arachnoid cysts of the posterior fossa frequently have abnormally high transverse sinuses. These children should be evaluated preoperatively by MR imaging to guide appropriate modification of the burr-hole placement. Frontal burr holes generally are placed along the coronal suture 2 to 3 cm from the midline (Fig. 1–2B). Anterior burr-hole placement may be preferable when endoscopic catheter placement or other procedures (e.g., septostomy, cyst fenestration) are planned.

INDICATIONS FOR CSF SHUNT INSERTION
PREOPERATIVE ASSESSMENT
Initial Presentation
Shunt Failure
OPERATIVE PLANNING
Shunt Selection
Preoperative Preparation
INTRAOPERATIVE TECHNIQUE
Ventriculoperitoneal Shunt
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


