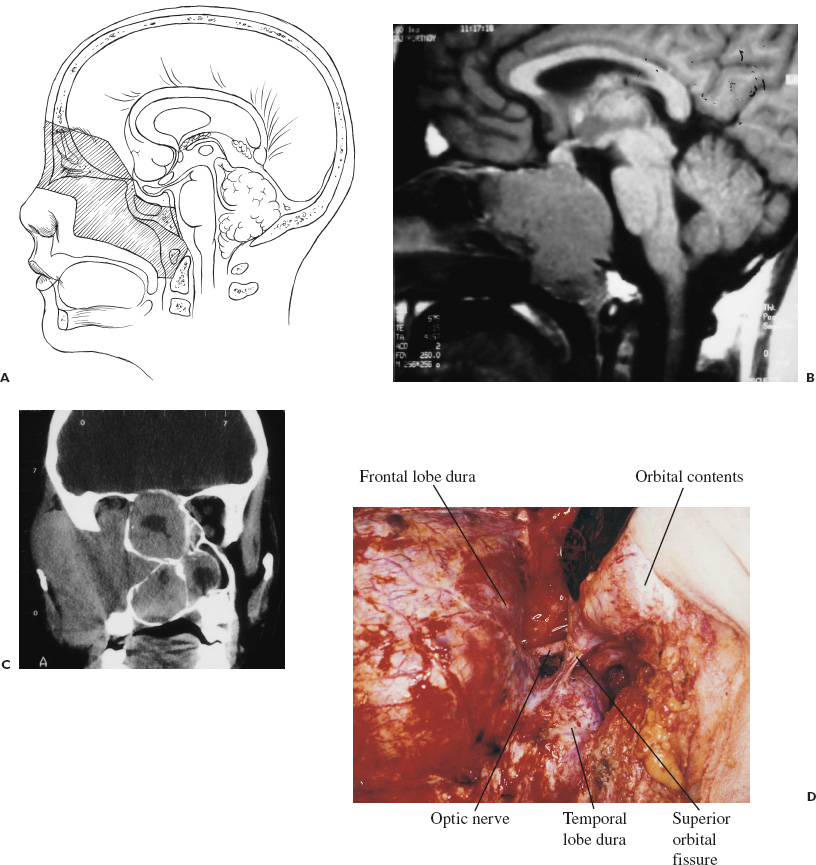
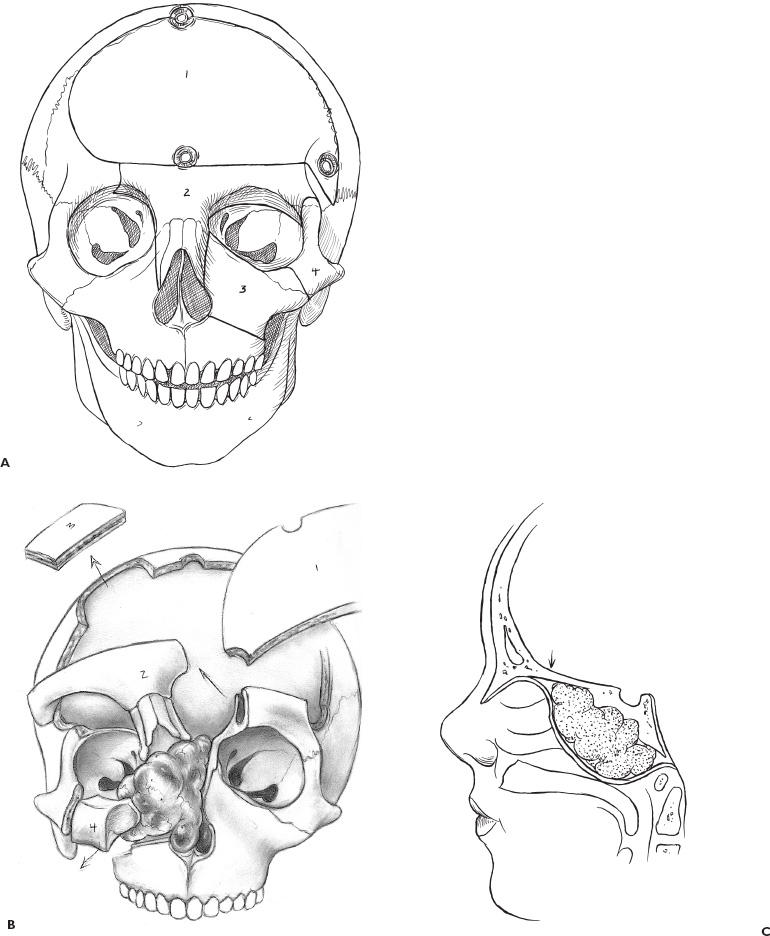
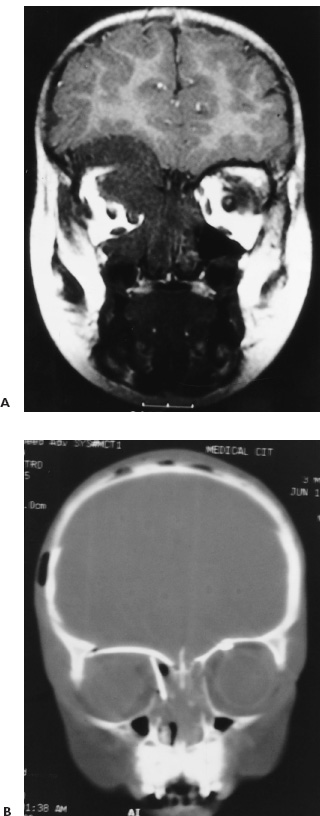
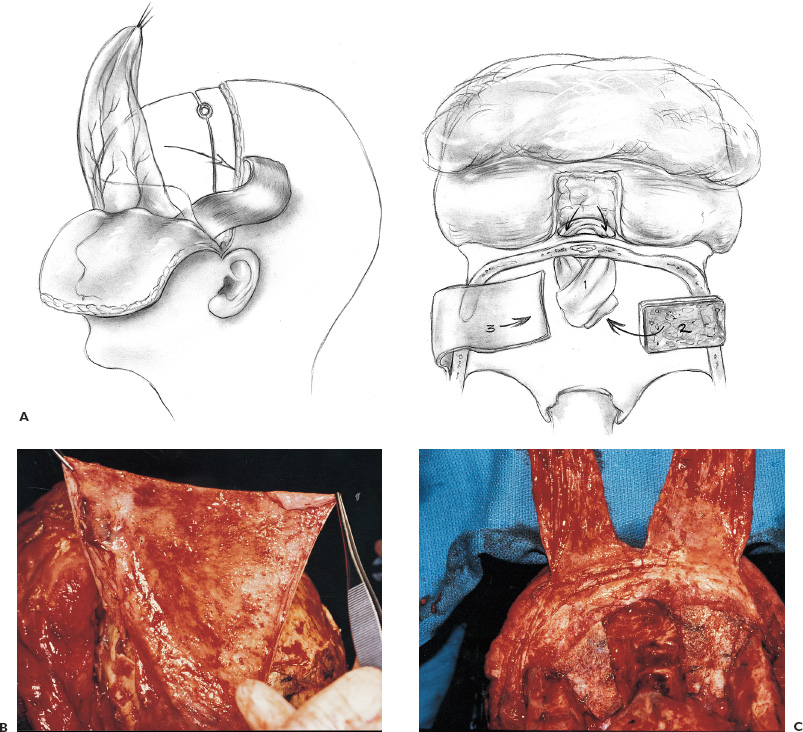
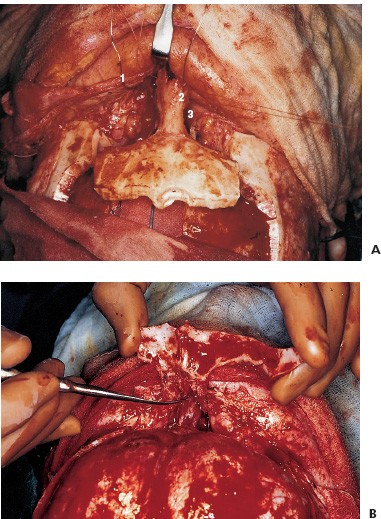
There is no single surgical approach to the skull base in children and the various approaches have been discussed elsewhere (Bruce, 1999). The most frequent lesions of the skull base in children are central and anterior in the frontal fossa, air sinuses, or clivus. The most common surgical approach is an extended subfrontal approach or a modification of this to focus on unilateral lesions. This is the procedure, with its various modifications, that is discussed in detail here. Areas that are accessible through an extended subfrontal approach or a modification include the orbit, sinuses (frontal, ethmoidal, sphenoidal, maxillary), nasal cavity, frontal fossa, pterygoid fossa, infratemporal fossa, and clivus. This approach has many advantages: a cosmetically invisible incision; the ability to reconstruct the face, orbits, and nose as well as the forehead; access to a large area of the skull base; and ready access to cranial bone for splitting to aid in the reconstruction. In addition, this approach under the frontal lobes minimizes brain retraction and allows both intradural and extradural access if necessary. It can be performed at almost any age of childhood and is appropriate for the treatment of many pathological lesions in this area. The extended subfrontal is appropriate for midline and paramedian lesions but also for bilateral orbital and cavernous sinus lesions (Fig. 14–1). An extended orbitofrontal approach is used for unilateral orbital and anterior middle fossa lesions (Figs. 14–1C and D). The primary step in deciding which operative procedure will be best is to identify the epicenter of the tumor. Because the tumor grows from this center, an approach that gives access to the epicenter is most likely to enable complete surgical excision. If the tumor is of extradural origin, the first operative procedure should be by an extradural approach. The extended subfrontal approach is not adequate for tumors that extend superiorly and posteriorly to the pituitary fossa because it is difficult, if not impossible, to see around the angle produced by the pituitary fossa. The use of the endoscope is of limited value unless the tumor is soft and easily aspirated with the sucker. (Lesions in this area require a Lafort 1 approach through the mid-face.) The remainder of the clivus can be accessed in cases where the tumor involves the sphenoid sinus as well as the clivus and posterior nasopharynx (Fig. 14–1B). The lateral extension of this exposure is also limited superiorly by the carotid arteries and the cavernous sinus. Inferior to the cavernous sinus, the lateral exposure is good. A final drawback to this approach is that, if the dura of the middle fossa has been eroded by the tumor, it is impossible to achieve a primary watertight repair. A pericranial or other flap must be used, which is a limitation of any approach to this area. Thus, as part of the surgical planning, an idea about the status of the middle fossa dura must be obtained from the imaging studies and an appropriate flap for transfer to the skull base planned. This approach can be combined with a facial degloving or maxillotomy approach to give better access to the facial structures, maxillary sinuses, nasal cavity, and upper jaw (Fig. 14–2). If maxillectomy and upper jaw resection are required, reconstruction below the level of the orbital floor can rarely be done as a primary procedure, and a temporary prosthesis is used to maintain the soft-tissue alignment. Preoperative plain photographs of the patient are helpful to achieve the best approximation to the preoperative ap pearance during the reconstructive phase of the operation. Often one orbit is involved and occasionally both by tumor. Reconstruction of the orbit is difficult (Fig. 14–3) and often results in enophthalmos or exophthalmos. Keeping as much as possible of the orbital roof and walls intact during the initial bone resection helps facilitate reconstruction. Neuroimaging studies usually consist of computed tomography (CT), magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA) to evaluate both soft-tissue and bone involvement. Angiography is done depending on the appearance of the tumor, its suspected vascularity, and the degree of compression of the carotid arteries seen on MRA. If the tumor appears extremely vascular, preoperative embolization of the extracranial supply is helpful to decrease blood loss. Surgery should be done within 24 hours of the embolization, if possible, especially if there is additional vascular supply from the internal carotid branches, because these vessels will enlarge over time and may be difficult to control during surgery. Preoperative radiation is rarely recommended because of tissue-healing problems that can occur postirradiation. For highly malignant tumors (e.g., rhabdomyosarcoma) or extensive tumors that are known to respond to chemotherapy or radiation (e.g., olfactory neuroblastoma), presurgical chemotherapy can be valuable for reducing the size of the tumor and to increase the chances of complete resection. Antibiotics are given at the induction of anesthesia, not before. Nasal cultures may be used to select antibiotic coverage, although we usually do not do this in children. The most frequently used antibiotic is cefotaxime (Claforan). The hair is usually washed the night before with Hibiclens shampoo. Following the induction of anesthesia and insertion of monitors, the nasal mucosa is injected with 0.25% bupivacaine hydrochloride (Marcaine) plus 1:200,000 epinephrine. The sublabial region is injected if a sublabial incision is likely to be required. The endotracheal (ET) tube needs to be well secured and in older children usually is wired to the front teeth. The location of the ET tube must be compatible with any potential surgical access requirements. The conjunctival sack is covered with lacrilube, and the lids are sutured closed to protect the cornea. Preparation is with Betadine soap and lotion and includes the entire head and face, including the mouth, if a sublabial incision is required. The nasal cavity is also prepared with Betadine lotion. The hair is parted along the incision line and held in place with rubber bands. The incision is made in a zigzag configuration, well behind the hairline, usually extending from ear to ear. The zigzag incision is cosmetically better. The scalp flap is reflected anteriorly in the subgaleal plane, keeping the pericranial layer intact if possible. Once the orbital rims are identified, a decision is made as to how the pericranial flaps should be based. This decision depends on the locus of the tumor and the extent of dural involvement. The usual configuration is shown in Figure 14–4. The flaps are brought into the frontal area through the burr holes in the lateral aspect of the frontal bone. The advantage of this configuration is that the flaps are left attached to the temporalis muscle, and the blood supply is likely to be better preserved than when the flaps are based frontally. In addition, the temporalis muscle can be reattached to the lateral orbit to avoid a cosmetically disfiguring temporal indentation. If the pericranial flaps are brought in frontocentrally, they often produce noticeable bulging in the medial epicanthal area, which usually resolves over several months and has not required a second operation for correction of the cosmetic deformity. FIGURE 14–4. Pericranial flaps. A: The pericranial flaps which can be based anteriorly or laterally. The second drawing shows the site of entry of the flaps into the intracranial space; either through a lateral burr hole or through two medially located drill holes. 1, Galeo-frontalis flaps over ethmoid. 2, Cribriform bone graft added. 3, Pericranial flap over cribriform bone graft. B: Operative photograph of a large pericranial flap based on the left temporalis muscle. This is large enough to cover the whole cranial base of the frontal fossa or to swing into the sphenoid sinus to cover the temporal lobe. C: Operative view of the reconstructed orbital roofs in a teenaged patient with bilateral optic nerve encroachment by fibrous dysplasia. In this case, two pericranial flaps have been prepared based frontally. A subperiorbital dissection is made in each orbit. If the supraorbital nerve is enclosed within a bony canal, a small osteotome is used to open the canal and spare the nerve. The subperiorbital dissection is carried superiorly, laterally to the lateral rim of the orbit and mesially. Mesially, the lacrimal duct must be identified and preserved along with the two heads of the medial canthus. The duct and lacrimal sac are positioned just posterior and lateral to the insertion of the heads of the medial canthal tendon. We have had more successful repositioning of the medial canthus since we started leaving it attached to the nasal bone and cutting out a small piece of the nasal bone on either side of the midline with the canthus connected to it (Fig. 14–5). The bifrontal bone flap is now outlined. The size of the flap depends on the amount of bone required for reconstruction and the extent of the tumor. The more posteriorly and laterally the tumor extends, the larger the frontal flap. The frontal craniotomy flap also may be extended into one middle fossa if the tumor is predominantly unilateral in the orbit or lesser wing of the sphenoid. A single posterior burr hole is placed over the sagittal sinus as far posterior as necessary (Fig. 14–2A). In children younger than 10 years of age, there is rarely a frontal sinus; thus, a frontal midline burr hole is optional. If one is made, it should be placed at least 4 cm above the nasion to preserve the strength of the bone of the bandeau. As many burr holes as necessary are placed to be able to free the dura and to avoid tearing it with the craniotome. The freeing of the dura usually is done using a Gigli saw guide. The frontal flap then is excised with the craniotome, trying to bevel the edge as much as possible. The greater the bevel, the better the fit when the bone is reattached. An epidural dissection is now made in the midline to free the dura entering the foramen caecum. The dura is freed laterally over the orbital roofs, medially to the lateral margins of the cribriform plate (Fig. 14–6) and posteriorly to the lesser wing of the sphenoid bone. The nasal dissection is carried below the nasion down to the junction of the nasal bone and the nasal cartilage. The cartilage is freed from the nasal bone, keeping the muccosa of the nose intact, if possible. Lateral saw cuts are made through the frontal bone and orbital roof. Posterior cuts are made along the medial aspect of the roof of the orbit, lateral to the cribriform plate (Fig. 14–6A). The more posteriorly the roof can be cut, the easier the reconstruction of the orbit. The ability to preserve the orbital roof depends on it not being invaded by tumor. The medial anterior skull base with the cribriform plate is left intact, and the anterior midline saw cut is made anterior to the crista galli. The anterior bandeau with the nasal bone can now be removed (Figs. 14–5A, B and Fig. 14–6B). If there are frontal sinuses within the bandeau, they must be cranialized. The nasal cavity is closed at the end of the procedure with a pericranial flap.
PREOPERATIVE CONSIDERATIONS
OPERATIVE TECHNIQUE
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


