Cervical MRI (axial plane and T1 fat suppression technique). (A) Vertebral artery dissection. (B) Bilateral internal carotid artery dissections. Arrows indicate semilunar signal hyperintensities representing intramural hematoma.
MRI has replaced invasive catheter angiography as the gold standard for extracranial dissections. Magnetic resonance angiography (MRA) or CTA are mainly used to localize the dissection site, demonstrate its extension and possible complications such as pseudoaneurysms. In occlusive dissections, both catheter angiography and MRA show aspecific findings and only MRI is able to show the cause. It is noteworthy that in dissected vessels of smaller diameter and/or tortuous course, specific MRI findings are less frequent due to the small dimensions of the mural hematoma and artifacts such as flow-related enhancement. Consequently, catheter angiography is still considered the most reliable diagnostic method in these instances (e.g., intracranial dissections).
However, when compared to MRI, ultrasound has some advantages:
1. In addition to local morphological signs, it documents vessel wall hemodynamics which may be suggestive of dissection and it assesses hemodynamic consequences in the intracranial circulation.
2. Ultrasound examination can be easily repeated: notably dissections are dynamic processes with extension of mural hematoma in the longitudinal and/or transversal plane, possibly turning from a normal finding to an occlusion of the vessel within a short time. This characteristic makes the sensitivity of any diagnostic method clearly time dependent and represents the main determinant of discrepant findings in different studies and compared to other imaging modalities performed later on. Therefore, a repeat exam performed the day after might disclose completely different findings and is strongly recommended in the case of initially normal results.
3. The real domain of ultrasound is to monitor the recanalization process, once the diagnosis is established and treatment started. This way the necessary duration of anticoagulant treatment can be guided by ultrasound follow-up exams [23].
Focused questions
While scanning patients with possible cervical artery dissection, there are a few key questions to focus on:
Are there morphological signs of dissection?
Are there hemodynamic signs of dissection?
Once the diagnosis has been made, the questions during follow-up are as follows:
Is there a partial/complete recanalization?
Is there a new vessel dissection?
Is there a recurrent dissection?
Scanning tips for the carotid system
The ultrasound investigation of patients with a clinical suspicion of carotid dissection should include a complete study of the anterior circulation: (1) a morphological and hemodynamic assessment of both CCAs and of the extracranial portion of the ICAs; and (2) a hemodynamic evaluation of the intracranial portion of the ICAs, of the middle cerebral arteries (MCAs), of the anterior cerebral arteries (ACAs) along with a documentation of collateral flow pathways.
High-frequency (5–10-MHz) linear transducers allow a detailed evaluation of the carotid wall in proximal cervical segments, but the distal parts of both internal carotids are not accessible with this approach. For evaluation of these segments, low-frequency (1.8–3.6-MHz) sector probes are used; yet these probes have a significantly lower spatial resolution at brightness mode (B-mode) and color Doppler imaging and therefore they have a lower chance of detecting directly the intramural hematoma. The intracranial vessels should be investigated via the transtemporal, submandibular and transorbital approaches with a phased array transducer (≥2 MHz).
Ultrasound findings of internal carotid artery dissection
There are two types of ultrasound findings in patients with ICAD: (1) morphological and (2) hemodynamic. Furthermore, these findings can be direct or indirect.
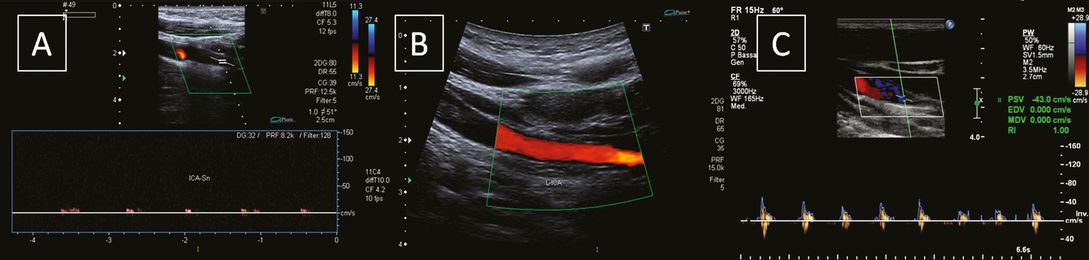
Typical direct morphologic findings (Figure 7.2) are hypoechogenic thickening of the vessel wall, luminal narrowing, echolucent intramural hematoma, double lumen, intimal flap or demonstration of a dissecting membrane, and pseudoaneurysm [24,25,26,27]. Pathognomonic signs (intramural hematoma, double lumen, intimal flap) are found in only about 25% of patients: in particular, the detection rate by ultrasound of an intramural hematoma, a double lumen and an intimal flap is about 15–25%, ≤2% and 2% respectively [14,19].
Internal carotid artery dissection: morphologic signs. (A) White arrow indicating a dissecting membrane; (B) distal tapering stenosis; (C, D) distal serpiginous stenoses. The white asterisks indicate a hypoechogenic thickening of the vessel wall representing an intramural hematoma.
Indirect morphological findings are normal carotid bifurcation or only mild atherosclerotic wall changes [5,25,28], distal tapering stenosis, increase of outer vessel diameter or circumference, and early thrombus detection [29].
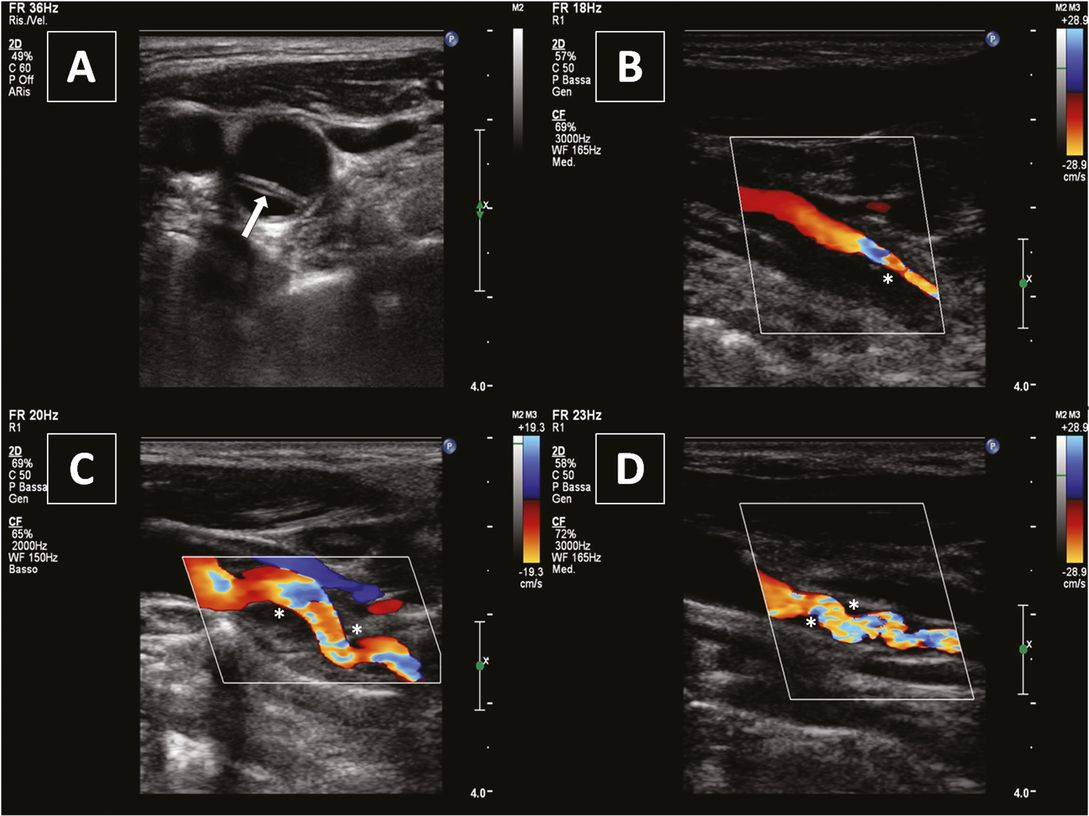
The most common finding of an ICAD (close to 90% of the patients) is a hypoechogenic wall thickening determining a stenosis/occlusion at a site not typically involved in atherosclerotic disease (Figure 7.3) [24,30].
Internal carotid artery dissection: hemodynamic signs. (A) Focal increase of blood flow velocities indicating a hemodynamic significant stenosis. (B) alternating to-and-fro stump signal indicating vessel occlusion.
Compared to atherosclerotic luminal narrowing, ICAD stenosis begins a few centimeters distal to the bifurcation and usually extends over a longer distance [19,25].
Therefore, in a patient with a clinical suspicion of ICAD, an examination of the carotid system cannot be said to be concluded if the distal part of the ICA has not been assessed, especially with a sector probe. In patients with fibromuscular dysplasia, a known risk factor for CAD [31], irregular wall thickening, multisegmental stenosis or an aberrant course of the ICA are frequently found [32,33]. Morphological criteria alone are diagnostic in only about 50% (20–63%), mainly due to inadequate direct imaging for retromandibular location.
Typical direct hemodynamic criteria are significant increase of blood flow velocities due to a distal stenosis, that is, high-cervical, retromastoidal stenosis. When assessing flow velocities the contralateral vessel is usually taken for comparison; in patients with CAD, one has to be careful with that strategy since up to 25% of patients have dissections affecting multiple vessels at the same time [7]. The combined use of morphological and hemodynamic criteria is diagnostic in 74–95% of ICAD patients and increases with repeated examinations [14,25,26,27].
When ICAD is located intracranially, only indirect hemodynamic criteria are detectable: prestenotic high-resistance flow profiles, poststenotic dampened flow profiles and intracranial collateral circulation [14].
Sensitivity of combined ultrasound techniques, regarding any abnormal finding, has in skilled hands a high sensitivity ranging from 80% to 96% [5,14,25,26,28,30,35,36,37]. Sensitivity, specificity, positive and negative predictive values for CCD diagnosis of patients with ICAD causing carotid territory ischemia were 96%, 94%, 92% and 97%, respectively [36]. Furthermore, different ultrasound techniques yield different sensitivities: 82% for CCD, 91% for power Doppler (PD) and 98% for B-flow [38]. B-flow significantly increased the sensitivity of ultrasound for dissections of neck vessels. Intimal flaps, fissures of membranes and residual flow within the true and false lumen were better detected by B-flow than by CCD and PD [39]. Compared to CCD and PD, B-flow has a better spatial resolution and no angle dependency of the probe during the examination. It is not based on the Doppler principle, so velocity measurements are not possible. One major advantage is the lack of color blooming or aliasing.
However, the diagnostic sensitivity is different for several ICAD subcategories: in patients having suffered ischemic events, the sensitivity of ultrasound was 96% declining to 71% in patients without ischemic events (painful Horner’s syndrome, cranial nerve palsies: IX–XII or more rarely III, IV, VI) [5,40]. Accordingly, patients with ICAD and ischemic events had significantly more high-grade stenoses and occlusions (83%) than those without ischemic symptoms (40%) [5].
False-negative findings on ultrasound are reported in 2.8–16% of all ICADs. They are mainly due to subadventitial dissections without lumen compromise or low-grade stenosis with mild mural hematoma located outside the vessel segments directly accessible to ultrasound [14,41] and are more frequently found in patients without cerebral ischemic signs [5]. Another limitation of ultrasound is the inability to demonstrate intracranial pseudoaneurysms [41].
Since a normal ultrasound exam does not exclude ICAD, the gold standard for diagnosis is MRI and MRA: they are able to show the pathognomonic intramural hematoma in more than 90% of cases. Yet, MRI/MRA and ultrasound are considered complementary methods since only ultrasound can show hemodynamic consequences.
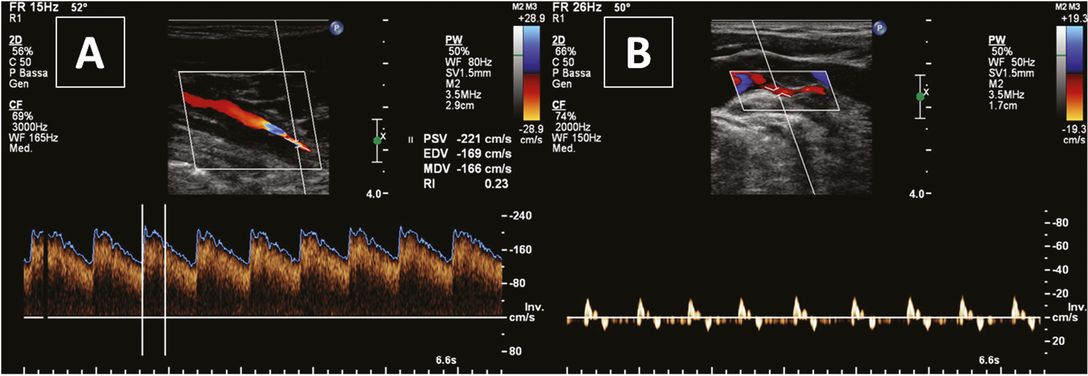
Ultrasound is the method of choice for follow-up investigations, since it is helpful to determine the time of recanalization; this way the necessary duration of anticoagulant treatment can be guided by ultrasound follow-up exams. Recanalization results from the resorption of the wall hematoma and from the resolution of the intraluminal thrombus within few weeks (Figure 7.4). The overall recanalization rate of ICAD is about 76%, while a complete recanalization has been reported in 55% of patients (58.7% in case of initial ICA stenosis, 40% in subjects with initial ICA occlusion). Most lumen changes occur within the first 6 months after dissection and they are only rarely seen (<1%) after 1 year [23]. High-grade (>80%) stenosis and occlusion were associated with less frequent recanalization [34].
Ultrasound monitoring of internal carotid artery dissection. (A) Near-occlusion; (B) partial recanalization after 1 month.
In very selected cases of carotid dissection associated with critical hemodynamic insufficiency or thromboembolic events that occur despite medical therapy, the patient undergoes endovascular stent placement; in these instances, ultrasound is useful in checking the efficacy of treatment in restoring vessel patency (Figure 7.5).
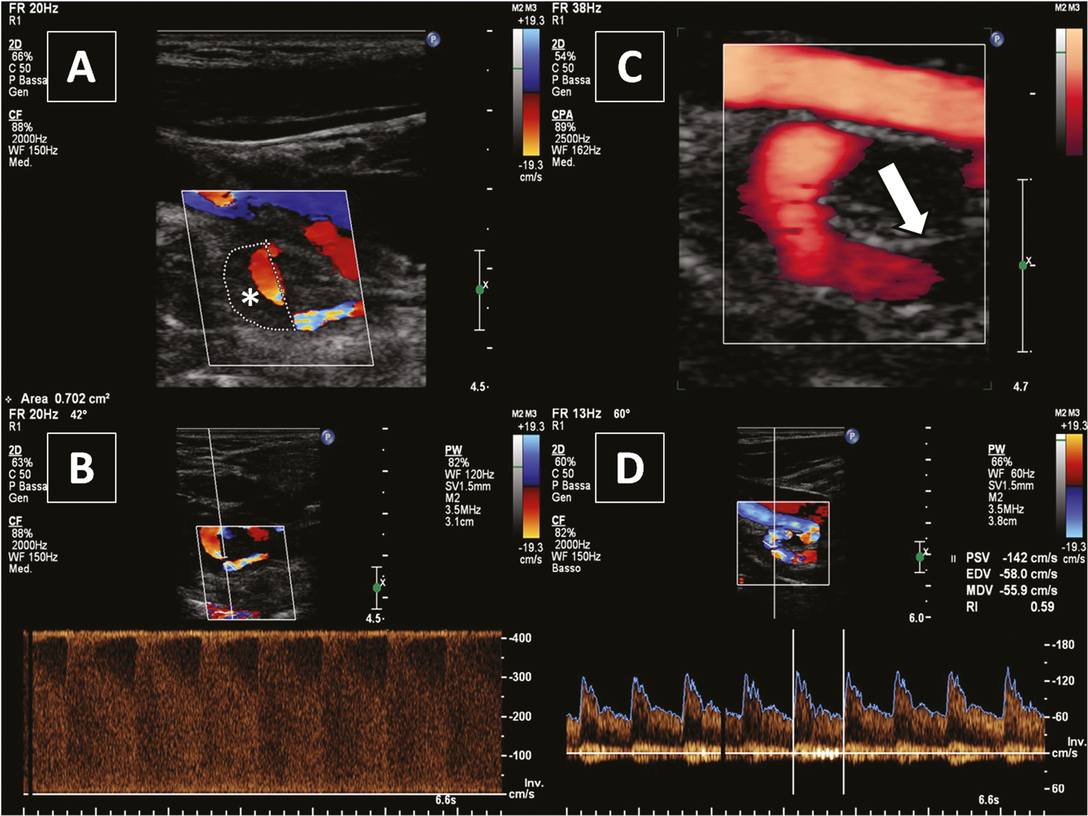
Internal carotid artery dissection pre and post endovascular treatment. (A) Large intramural hematoma (*) in a carotid coiling; (B) marked increase of blood flow velocities indicating a very tight stenosis; (C) white arrow indicating a stent; (D) recanalized vessel.
Ultrasound monitoring is also valuable for detecting recurrence of dissection (Figure 7.6). Two recent reports have shown that early recurrence rate is much higher than previously thought and varies from 19% to 26% in the acute phase of the disease [23,33]. Late recurrence is a more infrequent event, occurring only in 2.7% of patients [23].
Ultrasound findings of common carotid artery dissection
Common carotid artery dissection (CCAD) is a rare and poorly characterized cause of ischemic stroke. The estimated prevalence of CCAD is <1% of all cervical artery dissections. The reasons for the low frequency might be a different ultrastructure of the vessel wall: the ICA is a muscular artery, whereas the CCA is an elastic artery. The main difference between the two arteries is in the tunica media: in elastic arteries, the tunica media consists of mostly elastic fibers, whereas in the muscular arteries it consists mainly of smooth muscle tissue. Muscular arteries have two elastic layers in the tunica media (the external elastic lamina and internal elastic lamina), which may make them more susceptible to dissection [42].
CCAD can be traumatic, iatrogenic, spontaneous or associated with aortic dissection. It is the most common mechanism of ischemic stroke of type A thoracic aortic dissection (AAD), seen in 85% of cases in one series, more commonly on the right side [43].
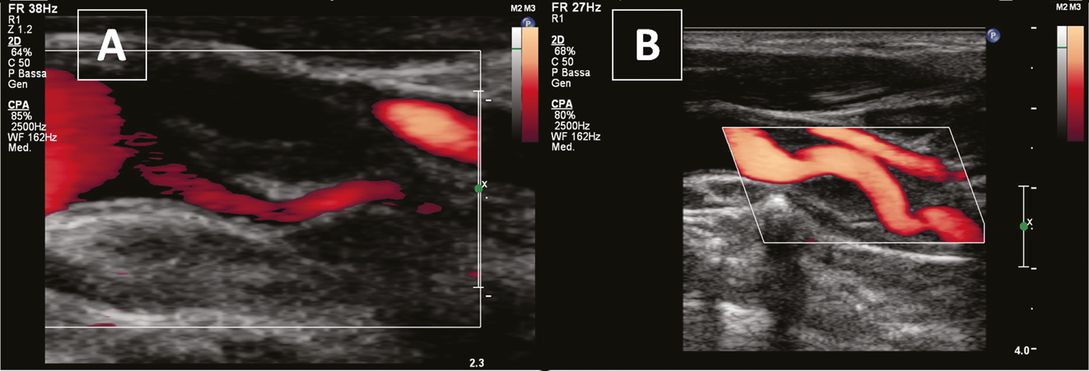
The most frequently reported neurosonographic findings (Figure 7.7) include a double lumen, mural thrombus, intraluminal hyperechoic/isoechoic lesion and intimal flap; more rare findings are carotid occlusion and pseudoaneurysm [4]. When urgent ultrasound is performed for suspected CCAD, axial views of the vessel should be acquired.
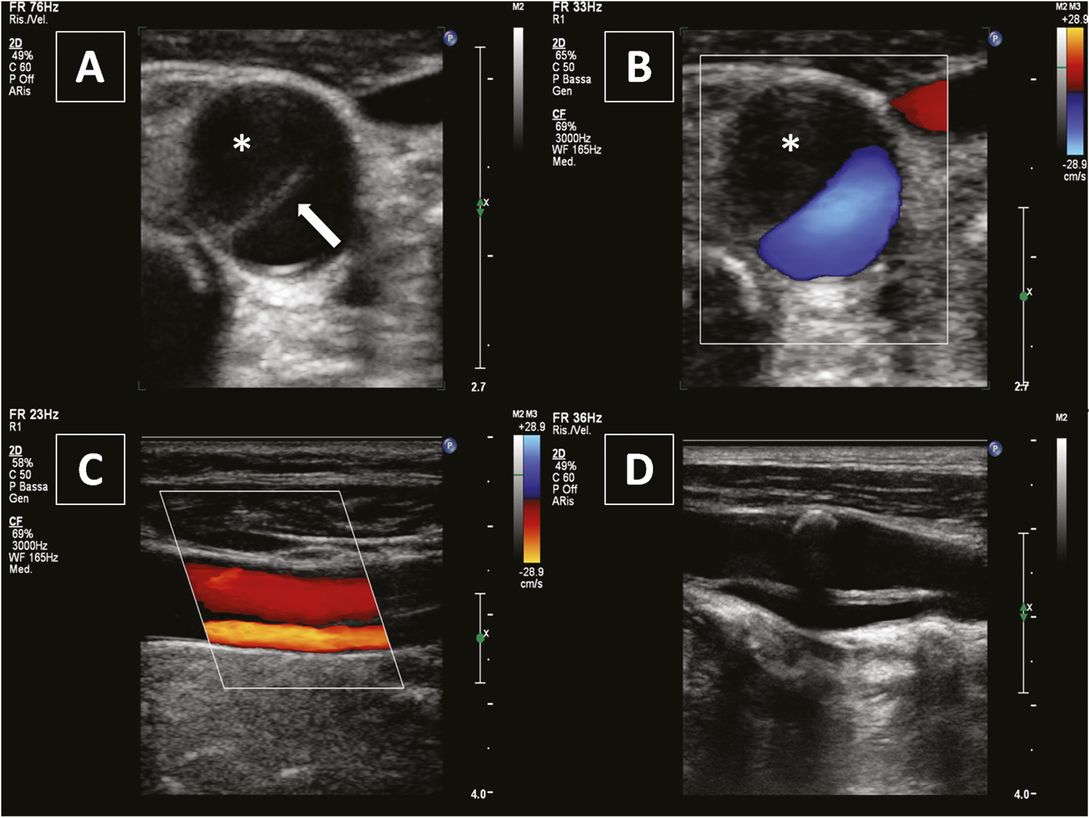
Common carotid artery dissection. (A) and (B) axial views showing a dissecting membrane (white arrow) and intramural hematoma (asterisk). (C) Longitudinal view showing a double lumen and a dissecting membrane. (D) Dissecting membrane extending into the internal carotid artery and a calcified plaque at the carotid bulb.
In cases of CCA occlusion, blood will usually flow from the ECA to the ICA, even though occasionally flow reversal may be seen with the ICA flowing retrograde and into the ECA. This dynamic information regarding flow within the ICA and ECA distal to the CCA occlusion provides complementary information for cerebral angiography and revascularization planning. Because of its association with aortic dissection, early recognition of CCAD might affect the decision regarding thrombolysis for acute ischemic stroke. Since thrombolysis is potentially harmful in patients with CCAD associated with AAD, a chest CT scan is mandatory to exclude an aortic dissection extending to the cerebroafferent vessels [44].
Scanning tips for the vertebral system
Similarly to the evaluation of the carotid system, the ultrasound investigation of patients with a clinical suspicion of VAD should include a complete study of the posterior circulation: (1) a morphological and hemodynamic assessment of the extracranial portion of both VAs: the origin (V0) and the tortuous V3 segment are better insonated by a low-frequency sector probe, while the extraforaminal segment (V1) and the intraforaminal segment (V2) are better studied by a high-frequency linear transducer; (2) a hemodynamic evaluation of the intracranial portion of the VAs (V4 segment), of the basilar artery (BA) and of the posterior cerebral arteries (PCAs) along with a documentation of collateral flow pathways. The intracranial vessels should be investigated via the transforaminal approach with a phased array transducer (≥2 MHz).
Ultrasound findings of vertebral artery dissection
The diagnostic criteria for VAD are basically the same as for ICAD. Analogous to ICAD, the most frequent ultrasound finding (present in about 80% of patients) is a stenosis or occlusion. However, due to the smaller caliber of the VA compared to the ICA, deeper location, a course in a segmented osseous canal with acoustic shadowing by the transverse vertebral processes, close vicinity to the vein and frequent unilateral hypoplasia, assessment of the VA is more difficult and reported findings more variable [45,46,47,48]. Namely, since the full length of the VA cannot be visualized at all times, sometimes it is not possible to show directly the site of the stenosis. Due to smaller vessel diameter and tortuous vessel course it is also difficult to differentiate between a dissection and an atherosclerotic stenosis (sensitivity = 75–92%), unless a typical sign of dissection is evident: that is, irregular stenosis, thickened hypo- or isoechogenic vessel wall indicating the presence of an intramural hematoma, or a double lumen [13,14,30]. Thickening of the vessel wall and a double lumen can be found in 10–20% of cases [14]. Just as for ICA, dissection and atherosclerosis occur at different sites: VA dissections occur primarily in the V2 and V3 segments, while VA atherosclerotic lesions are mostly found in the V0 and V4 segments [13].
Specific morphological findings (Figure 7.8) are especially reported if VAD is located in the V1 or V2 segment [27,46], but seem to be less frequent than in ICAD and especially rare in V3 dissections [48].
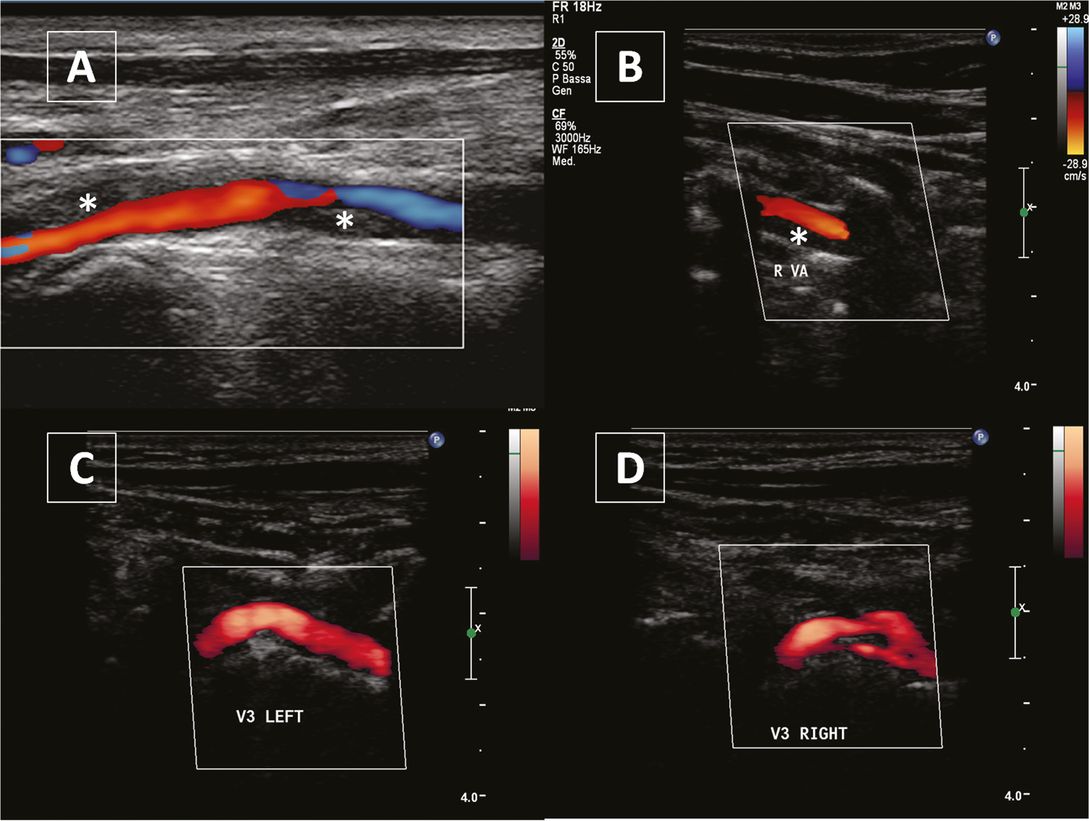
Vertebral artery dissection. (A) and (B) Hypoechogenic vessel wall thickening (asterisks) representing an intramural hematoma in the V1 segment and in the V2 segment respectively. (C) and (D) display a normal V3 segment and a double lumen in the dissected contralateral V3 segment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree