Cervical, Brachial, and Lumbosacral Plexi
Plexopathies are usually more difficult to recognize than lesions of individual peripheral nerves (peripheral neuropathies) or spinal roots (radiculopathies) because of the complex anatomy of the plexi. To localize a lesion accurately to a specific division of the plexus, the clinician must have mastered not only the anatomic intricacies of that division but also the motor and sensory supply of all peripheral nerve components supplied by the division.
This chapter reviews the anatomy of the cervical, brachial, and lumbosacral plexi and the localization of lesions within these plexi.
The Cervical Plexus
Anatomy
The cervical plexus, which is formed by the anterior primary rami of C1–C4 [53], is situated behind the sternocleidomastoid muscle and in front of the scalenus medius and levator scapulae muscles. It consists of a series of anastomotic loops situated near the spinal accessory (cranial nerve XI) and hypoglossal (cranial nerve XII) nerves. The branches of the cervical plexus may be divided into those that are predominantly sensory and those that are predominantly motor.
The cutaneous branches (Fig. 3.1), and their areas of sensory supply, include the following nerves:
1. The greater occipital nerve (C2): skin of the posterior scalp
2. The lesser occipital nerve (C2): skin of the mastoid process and lateral head
3. The great auricular nerve (C2–C3): skin of the lower cheek over the mandible, the lower part of the external ear, and the upper neck below the external ear
4. The transverse colli (cutaneous cervical nerves) (C2–C3): skin on much of the neck, especially the anterior neck
5. The supraclavicular nerves (C3–C4): skin immediately above the clavicle
6. It should be noted that there is no dorsal root from C1; therefore, C1 is a purely motor root.
The muscular branches of the cervical plexus (Fig. 3.2) include the following nerves and branches:
1. The ansa hypoglossi, which is a loop formed by fibers from the C1 root (the descending hypoglossal rami that course downward in company with the hypoglossal nerve proper) joining fibers from the C2 and C3 roots; the fibers of the ansa are distributed to the infrahyoid muscles (i.e., sternohyoid, omohyoid, sternothyroid, thyrohyoid, and geniohyoid), which aid in head flexion.
2. The phrenic nerve (C3–C5), which innervates the diaphragm.
3. Branches to the middle scalene and levator scapulae (C3–C4), which are essentially a lateral flexor of the neck and a rotator of the scapula, respectively.
4. Branches to the accessory nerve (cranial nerve XI), which supply the sternocleidomastoid (C2) and trapezius (C3–C4) muscles along with the accessory nerve proper.
Lesions of the Cervical Plexus
Injuries to the cervical plexus are infrequent, but any of its branches can be injured by penetrating wounds, surgical injury (e.g., carotid endarterectomy or radical dissection of malignancy), or various mass lesions. Involvement of the cutaneous branches results in altered sensation (e.g., sensory loss, paresthesias, or pain) in the distribution of these branches (e.g., after a lesion of the great auricular nerve, there is loss of sensation over the mandible and lower external ear). When the muscular branches of the cervical plexus are injured, there is weakness of the infrahyoid and scalene muscles (anterior and lateral head flexion), the levator scapulae (scapular rotation), and, to some degree, the trapezius (shoulder elevation) and sternocleidomastoid (head rotation and flexion) muscles as well. The muscles affected and the degree of paresis depend on the specific branch of the cervical plexus that is injured.
Individual branches of the cervical plexus may be damaged. The greater auricular nerve is most commonly damaged during surgery to the neck or face (e.g., face lift or parotid surgery) or during carotid endarterectomy [26,114]. Unilateral or bilateral greater auricular nerve damage may occur after hanging in suicide attempts (numb ear in resurrection) [6]. This nerve may also be injured by tumors and after cardiac pacemaker insertion [6]. The greater occipital nerve may be compressed or entrapped in its course through the muscles of the neck, especially the semispinalis and trapezius, or damaged by trauma or neurofibroma. The lesser occipital nerve may be injured during surgical procedures involving the posterior cervical triangle or by lacerations.
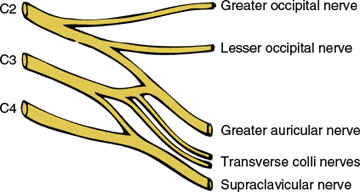
FIG. 3.1. Sensory branches of the cervical plexus.
Injuries to the phrenic nerve (C3–C5) deserve special consideration. Unilateral or bilateral damage to the phrenic nerve is more often caused by a mediastinal process than by damage to the cervical plexus itself. Paralysis of this nerve results in loss of diaphragmatic movement on the affected side. When unilateral, this paralysis results in little disability at rest, but dyspnea may occur with exertion. On the affected side, the diaphragm fails to descend with inspiration and may paradoxically be drawn upward. Bilateral phrenic lesions may result in prominent exertional dyspnea and severe alveolar hypoventilation with hypocapnia. Occasionally, the phrenic nerve receives an anastomotic branch from the subclavian nerve, in which case diaphragmatic action may be normal after a proximal phrenic lesion.
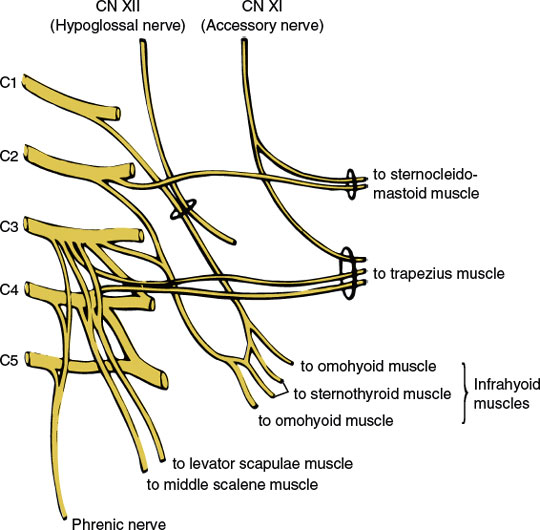
FIG. 3.2. Motor branches of the cervical plexus.
Unilateral or bilateral phrenic nerve paralysis may occur in isolation (idiopathic diaphragmatic paralysis) or with more diffuse motor involvement as part of the Parsonage–Turner syndrome (neuralgic amyotrophy) [9,77,135]. Lin et al. reported a painless paralysis of the diaphragm caused by bilateral phrenic neuropathies with relatively acute onset and without antecedent factors such as infection or prior surgery thought to have occurred on an immune basis [85]. The phrenic nerve may also be damaged during operations in the neck or chest (e.g., open-heart surgery) or be compressed by aortic aneurysms, intrathoracic neoplasms, or enlarged mediastinal nodes [42,57,93,125,153]. The nerve may be injured in the neck during subclavian vein or internal jugular vein catheterization or as a complication of an indwelling central venous catheter [4,109]. Two patients developed unilateral diaphragmatic paralysis from phrenic nerve injury after minor cervical trauma, in one case following cervical chiropractic manipulation and in the other after a motorcycle accident [96].
Neck metastases, usually from breast cancer, may involve the phrenic nerve along with the sympathetic chain and recurrent laryngeal nerve, resulting in phrenic palsy associated with an ipsilateral Horner syndrome (miosis, ptosis) and ipsilateral vocal cord paralysis (Payne syndrome) [104]. Phrenic nerve injury (unilateral or bilateral) may complicate coronary artery bypass surgery, perhaps induced by hypothermia, nerve stretch, or internal mammary artery dissection or harvesting [12,27,79,142]. During liver transplantation, the phrenic nerve may be traumatized when it is inadvertently clamped along with the inferior vena cava [12]. Other causes of phrenic neuropathy include amyotrophic lateral sclerosis, diabetes mellitus, mediastinal radiation therapy, sarcoidosis, tuberculosis, herpes zoster, Lyme disease, Charcot Marie Tooth disease type 2C, multifocal motor neuropathy, critical illness polyneuropathy, chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), and Guillain–Barré syndrome [12,13,28,46,58,120,123,151]. An acute, isolated bilateral phrenic neuropathy caused by sarcoidosis has also been described in a patient who had enlarged mediastinal lymph nodes and abnormal numbers of white blood cells in CSF [110].
The Brachial Plexus
Anatomy
The brachial plexus (Fig. 3.3) is formed from the anterior primary rami of the segments C4, C5, C6, C7, C8, and T1 [53]. A communication branch between the T2 root and the brachial plexus is also common [87]. The plexus is approximately 15 cm long in adults and extends from the spinal column to the axilla. It is divided into five major components (in a proximal to distal direction): roots, trunks, divisions, cords, and branches (the mnemonic Robert Taylor Drinks Cold Beer serves as a means of remembering the names and order of these components).
The fifth and sixth cervical roots course downward between the scalenus medius and anterior muscles and unite to form the upper trunk of the plexus. The seventh cervical root also inclines downward between the scaleni and, at the lateral border of the scalenus anterior, emerges as the middle trunk of the plexus. The eighth cervical and first thoracic spinal roots unite behind a fascial sheet (Sibson’s fascia) and beneath the subclavian artery from the lower trunk of the plexus.
The three trunks traverse the supraclavicular fossa protected by the cervical and scalene musculature through most of their course. Lateral to the first rib, where the three trunks are located behind the axillary artery, they separate into three anterior and three posterior divisions. The three posterior divisions unite behind the axillary artery to form the posterior cord. The anterior divisions of the upper and middle trunks (C5–C7) unite to form the lateral cord, whereas the anterior division of the lower trunk (C8-T1) forms the medial cord. The cords pass through the space formed by the first rib and clavicle (thoracic outlet) and then give off the major terminal branches (peripheral nerves).
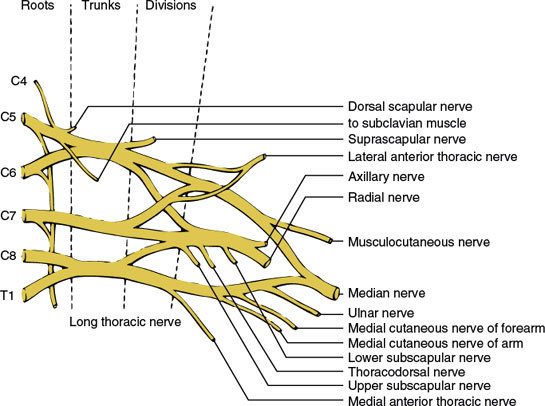
FIG. 3.3. The brachial plexus.
The major branches of the brachial plexus and the site of origin of these branches are as follows.
BRANCHES ORIGINATING FROM THE SPINAL ROOTS
The distribution of these individual nerves is given in Chapter 2. The long thoracic nerve arises directly from C5–C7 roots and descends vertically behind the plexus to innervate the serratus anterior muscle. The nerve to the subclavian muscle arises from the C5 and C6 roots and travels anterior to the plexus to innervate the subclavian muscle. The dorsal scapular nerve arises from the C4 and C5 roots and innervates the levator scapulae and rhomboid muscles.
BRANCH ORIGINATING FROM THE TRUNK OF THE BRACHIAL PLEXUS
The suprascapular nerve (C5–C6) arises from the upper trunk near its origin and innervates the supraspinatus and infraspinatus muscles.
BRANCH ORIGINATING FROM THE DIVISIONS OF THE BRACHIAL PLEXUS
The anterior thoracic nerves (C5–T1) (also called the pectoral nerves) consist of the lateral anterior thoracic nerve (C5–C7), which arises from the anterior divisions of the upper and middle trunks of the plexus, and the medial anterior thoracic nerve (C8–T1), which is a branch of the medial cord of the plexus. They supply the pectoralis major and minor muscles.
BRANCHES ORIGINATING FROM THE CORDS OF THE BRACHIAL PLEXUS
The distribution of these individual nerves is given in Chapter 2. Branches of the lateral cord consist of (a) the musculocutaneous nerve (C5–C7) and (b) the lateral head of the median nerve (C5–C7). Branches of the medial cord consist of (a) the medial anterior thoracic nerve (C8–T1), (b) the medial cutaneous nerve of the arm (C8–T1), (c) the medial cutaneous nerve of the forearm (C8–T1), (d) the ulnar nerve (C7–T1), and (e) the median head of the median nerve (C8–T1). Branches of the posterior cord consist of (a) the subscapular nerve (C5–C7), (b) the thoracodorsal nerve (C5–C8), (c) the axillary nerve (C5-C6), and (d) the radial nerve (C5–C8).
There may be considerable anatomic variation of the brachial plexus. In the prefixed plexus, all the components are shifted up one segment, resulting in a major contribution from the fourth cervical nerve. In the postfixed plexus, all the components are shifted down one segment, resulting in little or no contribution to the plexus from the fifth cervical nerve and a distinct contribution from the second thoracic nerve. These “one-level” variations in innervation occur in 3%–5% of patients and must be considered in any patient who does not fit the usual clinical presentation of a brachial plexopathy.
Lesions of the Brachial Plexus
Brachial plexopathies, in general, are usually incomplete and characterized by muscle paresis and atrophy, loss of muscle stretch reflexes, sensory changes (usually patchy and incomplete), and often, shoulder and arm pain (usually accentuated by arm movement). The most prominent sign of a brachial plexopathy is a clinical deficit that involves more than one spinal or peripheral nerve.
Brachial plexopathies are common and may present with a multiplicity of clinical syndromes that vary with the component of the plexus involved and the location of the lesion. Trauma is the most frequent cause of damage and may occur as a penetrating or closed injury (traction, avulsion, compression, or stretch) [29,39]. Traumatic stretch injuries are frequent consequences of motor vehicle accidents, especially in circumstances in which the victim is propelled from the vehicle (e.g., motorcycle, snowmobile, all-terrain vehicle, or boat). Gunshot wounds, lacerations, birth trauma, fracture—dislocations of the shoulder, and orthopedic shoulder surgeries are other common situations associated with brachial plexus injury [5,70,71,99,130]. Brachial plexopathy in birth trauma is most commonly noted in children presenting in the vertex position, where progression of the shoulder is blocked by the symphysis, causing traction of the brachial plexus (shoulder dystocia) [34]. The main risk factor for shoulder dystocia is macrosomia, which occurs in maternal diabetes. Repetitive arm use in baseball pitchers may cause pain and numbness in the arm likely because of a plexopathy [86]. Certain “traumatic” plexopathies may occur with a delayed onset (hours or weeks) because of neurovascular injury, with subsequent expanding hematoma or pseudoaneurysm [107]. The usual precipitants are fracture—dislocation of the humerus, gunshot wounds, and axillary artery trauma associated with medical procedures (especially orthopaedic procedures). Brachial plexus damage has also been described after reduction mammaplasty [7], subclavian vein catheterization [66,106], thoracoscopic sympathectomy [81], and thoracoscapular fusion in facioscapulohumeral muscular dystrophy [152].
Infraclavicular brachial plexopathy is a component of the medial brachial fascial compartment syndrome, a potential complication of percutaneous axillary vessel puncture during axillary angiography or axillary regional block [17,133,134]. This syndrome consists of pain, weakness, and numbness during or following the percutaneous procedure and involves the infraclavicular brachial plexus, most often the median nerve alone, followed by combinations of median, ulnar, radial, and musculocutaneous nerve involvement. The syndrome is often caused by hematoma formation within the medial fascial brachial compartment and requires urgent surgery.
The brachial plexus is probably the most susceptible of all nerve groups to damage from poor positioning during anesthesia (positioning trauma), perhaps because of its long mobile course and proximity to bony structures [25]. Stretch of nerve fibers, rather than compression, is the chief cause of injury [25]. Usually, these plexopathies present with painless motor deficits, especially affecting the C5–C7 levels [16]. Upper plexus lesions occur with full arm abduction, whereas lower plexus lesions occur primarily when the arm is close to the side. Abnormal arm positioning during alcohol intoxication or coma may also damage the plexus [118].
Other causes of brachial plexopathy include serum- and vaccine-induced lesions, radiation injuries, infections and toxic causes, mass lesions (e.g., neoplasms and hemorrhage from anticoagulants), systemic lupus erythematosus, heroin addiction, CIDP, and hereditary disorders [11,18,97,112]. A brachial plexopathy (probably immune mediated) has been described secondary to botulinum toxin injection for torticollis [47,126], following intra-arterial administration of cisplatin chemotherapy [63], and after intravenous high-dose cytarabine chemotherapy [116].
Inherited brachial plexus neuropathy has also been described (autosomal dominant inheritance), with recurrent episodes of brachial plexus neuropathy occurring in multiple family members [15,20,30,44,62,149]. The brachial plexus may also be involved in approximately 10% of patients with hereditary neuropathy with liability to pressure palsies, also an autosomal dominant condition, and recurrent brachial plexopathy may be the only symptom of entrapment in some families [8,19,95,100,148,149]. A variant of multifocal motor neuropathy in the brachial plexus may present with progressive arm weakness and tonic hand spasm [137].
Brachial plexopathy (radiculoplexopathy) may occur as a complication of coronary artery bypass graft surgery or cardiac valve replacement and usually affects the lower trunk or medial cord fibers [55,79,84]. Because there is a correlation between the site of jugular vein cannulation and the affected side in most cases, needle trauma is thought to play a role [79]. With open-heart surgery through median sternotomy, bilateral (although asymmetric) brachial plexopathies may occur, with pain being a prominent feature [50]. Brachial plexus injury may also follow liver transplantation [67].
Primary tumors of the brachial plexus (i.e., schwannomas, neurilemomas, hemangiomas, and neurinomas) are rare and usually present with a slowly growing swelling in the supraclavicular fossa or axilla, with little motor or sensory disabilities (except occasional pain) noted [10,88,101,108,117]. In a series of 25 patients with primary brachial plexus tumors, the presenting signs and symptoms included palpable mass (60%), numbness or paresthesias (44%), radiating pain (44%), local pain (16%), and weakness (12%) [10]. Localized hypertrophic neuropathy of the plexus, with progressive upper limb neurologic deficits, may rarely occur [119]. The plexus is more often affected by metastases (most frequently from breast cancer) or by direct infiltration from neighboring neoplasm, especially from carcinoma of the upper lobe of the lung (Pancoast tumor). With the latter lesions, the lower brachial plexus is initially affected, resulting in pain in the ulnar side of the hand, forearm, and arm, followed by other sensory symptoms and then by motor symptoms and Horner syndrome.
Patients with neurofibromatosis type I (NF 1) who develop pain or new neurological symptoms should have a rapid and thorough assessment for malignancy. The more extensive plexiform neurofibromas produce neurological complications in 27%–43% of patients with NF1 and may undergo malignant degeneration in 5% of cases. This point was illustrated by a patient with NF1 who developed a brachial plexopathy presenting with acute shoulder pain and weakness due to malignant degeneration of a plexiform neurofibroma of the plexus [102].
Brachial plexus lesions may also occur months to years after radiotherapy, usually for breast cancer and Hodgkin’s lymphomas [72,73]. It is often difficult to distinguish a plexopathy due to radiotherapy from that due to recurrent neoplasm or metastases. The clinical presentation may help distinguish these two causes of plexopathy [73]. With metastatic disease, the lower trunk (C8–T1) is predominantly or exclusively involved, severe pain is present at onset, and Horner syndrome is common. With radiation-induced plexopathy, the upper trunk or entire plexus is predominantly affected, paresthesias and weakness are more prominent than pain at the onset, and progressive lymphedema of the arm is more common. The numbness often involves the lateral aspect of the arm, and there is weakness of the shoulder girdle muscles. Some clinicians, however, have found no difference between the anatomic distribution of brachial plexus involvement in patients with neoplastic plexopathy and in those with radiation-induced plexopathy [52]. In both groups, weakness involved the muscles innervated predominantly by the lower trunk or the entire plexus. Patients with neoplastic plexopathy had a (a) higher frequency of pain as the initial and predominant symptom, (b) shorter duration of symptoms before diagnosis, and (c) higher incidence of Horner syndrome than patients with radiation-induced plexopathy [52]. In a study of radiation plexopathy in breast cancer, the entire plexus was affected in 50%, the upper trunk in 18%, the lower trunk in 4%, with assessment of level not clinically possible in 28% [98]. In these patients, symptoms often started during or immediately after radiation therapy, with numbness or paresthesias and pain being the most prominent symptoms. In another study of radiation plexopathy in patients with breast cancer, however, the latency to onset of symptoms was 1 month to 15 years, with motor deficits, pain, and paresthesias as the initial symptoms at presentation [36]. An acute ischemic brachial plexopathy from occlusion of the subclavian artery may occur as a late complication of radiation therapy [45]. This disorder is predominantly motor, sudden in onset, and painless, with paresthesias often felt in the forearm and hand. An acute reversible brachial plexopathy has been reported in some patients shortly after radiation therapy for breast cancer [113]. The symptoms generally occurred approximately 4 months after radiation therapy and were characterized by mild shoulder pain and arm paresthesias with severe but reversible arm weakness. Radiation-induced malignant and atypical peripheral nerve sheath tumors may also affect the brachial plexus and may be difficult to differentiate from tumor recurrence or radiation plexopathy [40]. A paraneoplastic brachial plexopathy may occur in patients with Hodgkin’s disease, especially following radiation therapy [75,105]. Finally, in one case, dystrophic calcification, a heterotopic formation of calcium in soft tissue, caused entrapment of the posterior cord of the brachial plexus with an onset many years after surgery and radiation therapy [90].
Neuralgic Amyotrophy
Parsonage–Turner syndrome [35] is a disorder characterized by acute, severe pain located in the shoulder and radiating into the arm, neck, and back. To prevent pain, movement of the arm is avoided and it is held in a position of flexion at the elbow and adduction at the shoulder (the flexion–adduction sign) [141]. The pain is followed within several hours to days by paresis of the shoulder and, predominantly, proximal arm musculature. Sensory loss can occur but is generally not marked. The muscles innervated by the axillary, suprascapular, radial, musculocutaneous, and long thoracic nerves are most commonly affected. Unilateral or bilateral phrenic nerve paralysis may occur [77]. In fact, in a series of 33 patients diagnosed with idiopathic phrenic neuropathy, 17 patients had clinical features of neuralgic amyotrophy [132]. The pain usually disappears within several days and bilateral (usually asymmetric) involvement may occur. The process is thought to be a brachial plexitis or multiple mononeuritis and is usually idiopathic, but may follow viral illness, immunizations, surgery, or childbirth [80,89,91]. Hereditary neuralgic amyotrophy is an autosomal dominant disorder with recurrent, episodic, painful, brachial neuropathy sometimes associated with characteristic features such as hypotelorism, short stature, and cleft palate [62].
The symptoms, course, and prognosis of neuralgic amyotrophy in a large group of patients with idiopathic neuralgic amyotrophy (INA, n = 199) and hereditary neuralgic amyotrophy (HNA, n = 47) was reviewed by van Alfen and van Engelen [136]. Generally, the course of the pain manifests itself in three consecutive phases with an initial severe, continuous pain lasting for 4 weeks on average. Sensory involvement was quite common and found in 78.4% of patients but was clinically less impairing than the initial pain and subsequent paresis. As a typically patchy disorder, INA was found to affect almost any nerve in the brachial plexus, although damage in the upper and middle trunk distribution with involvement of the long thoracic and/or suprascapular nerve occurred most frequently (71.1%). The authors found no correlation between the distribution of motor and sensory symptoms. In INA recurrent attacks were found in 26.1% of the patients during an average 6-year follow-up. HNA patients had an earlier onset (28.4 vs 41.3 years), more attacks (mean 3.5 vs 1.5), and more frequent involvement of nerves outside the brachial plexus (55.8% vs 17.3%) than INA patients, and a more severe maximum paresis, with a subsequent poorer functional outcome. In males the initial pain tended to last longer than it did in females (45 vs 23 days). In females the middle or lower parts of the brachial plexus were involved more frequently (23.1% vs 10.5% in males), and their functional outcome was worse. Overall recovery was less favorable than usually assumed, with persisting pain and paresis in approximately two-thirds of the patients who were followed for 3 years or more [136].
Total Plexus Paralysis
Total plexus paralysis is a rare syndrome that is usually due to severe trauma (usually a fall from a moving vehicle) and is characterized by the following signs:
Motor Signs. The entire arm is paralyzed and hangs limp at the patient’s side. All the arm’s musculature may undergo rapid atrophy.
Sensory Signs. There is usually complete anesthesia of the arm distal to a line extending obliquely from the tip of the shoulder down to the medial arm halfway to the elbow.
Reflex Signs. The entire upper extremity is areflexic.
UPPER PLEXUS PARALYSIS (ERB–DUCHENNE TYPE)
Upper plexus paralysis (Erb–Duchenne type) is a lesion that results from damage to the fifth and sixth cervical roots or the upper trunk of the brachial plexus. It is a common deficit and is usually due to forceful (traumatic) separation of the head and shoulder but may also be due to pressure on the shoulder (e.g., knapsack paralysis, rucksack paralysis, cadet palsy, or pack paralysis), firearm recoil [140], birth injury [5,34,103], and idiopathic plexitis (“neuralgic amyotrophy” or Parsonage–Turner syndrome). Sudden forceful depression of the shoulder during contact sports, especially football, may cause a transient episode of abrupt, intense burning dysesthesia and anesthesia involving one entire upper extremity, usually accompanied by generalized limb weakness (burners or stingers) [38,54,144]. The symptoms usually resolve in minutes without neurologic residual. Although symptoms in this condition involve the entire limb, findings are most prominent in the distribution of the upper trunk of the plexus [54,144]. Other sports reported to cause this syndrome include wrestling, hockey, basketball, boxing, and weight lifting [38].
The upper plexus syndrome consists of the following signs:
Motor Signs. The muscles supplied by the C5–C6 roots are paralyzed or paretic and atrophic. These include the deltoid, biceps, brachioradialis, and brachialis, and occasionally, the supraspinatus, infraspinatus, and subscapularis as well. The position of the limb is characteristic—the limb is internally rotated and adducted and the forearm is extended and pronated, the palm therefore facing out and backward. This is the so-called policeman’s tip or porter’s tip position. Shoulder abduction (deltoid and supraspinatus), elbow flexion (biceps, brachioradialis, brachialis), external rotation of the arm (infraspinatus), and forearm supination (biceps) are impaired. Very proximal lesions may also cause weakness of the rhomboids, levator scapulae, serratus anterior, and scalene muscles.
It has been noted that in some cases of obstetric brachial plexopathy, injured phrenic nerve, or C3–C5 roots may sprout into the adjacent injured upper and middle trunks of the brachial plexus. This aberrant regeneration produces co-contraction of the diaphragm and proximal limb muscles, resulting in the phenomenon referred to as respiratory synkinesis or the breathing arm [41]. This reinnervation may not be limited to the upper cervical roots because cases have been described of respiratory synkinesis selectively affecting intrinsic hand muscles (breathing hand) [41]. It is proposed that aberrant regeneration from upper thoracic roots and their intercostal nerves may produce respiratory synkinesis, resulting in the “breathing hand” [41].
Sensory Signs. Sensation is usually intact, but there may be some sensory loss over the outer surface of the upper arm, especially over the deltoid muscle.
Reflex Signs. The biceps and brachioradialis reflexes are depressed or absent.
Middle Plexus Paralysis
Lesions of the middle trunk or the corresponding individual anterior primary ramus of the seventh cervical root are rare but occur occasionally with trauma. The seventh cervical fibers to the radial nerve are primarily involved, and therefore the extensors of the forearm, hand, and fingers are paretic (including the triceps, anconeus, extensor carpi radialis and ulnaris, extensor digitorum, extensor digiti minimi, extensor pollicis longus and brevis, abductor pollicis longus, and extensor indicis). Forearm flexion is spared because the brachioradialis and brachialis are innervated predominantly by the fifth and sixth cervical segments. The triceps reflex may be depressed or absent, and a sensory defect, although inconsistent and often patchy, may occur over the extensor surface of the forearm and the radial aspect of the dorsum of the hand.
LOWER PLEXUS PARALYSIS (DEJERINE-KLUMPKE TYPE)
The lower type of brachial plexopathy (Dejerine-Klumpke type) results from injury to the eighth cervical and first thoracic roots or the lower trunk of the plexus. It is usually the result of trauma, especially arm traction in the abducted position, but is also seen after surgical procedures and is associated with lung tumors (e.g., Pancoast tumor) or other mass lesions (e.g., aneurysms of the aortic arch). The lower plexus syndrome consists of the following signs:
Motor Signs. All the musculature supplied by the eighth cervical and first thoracic roots are paretic and eventually atrophic. Therefore, there is weakness of wrist and finger flexion and weakness of the intrinsic hand muscles. Often, a claw hand deformity is evident.
Sensory Signs. Sensation may be either intact or lost on the medial arm, medial forearm, and ulnar aspect of the hand.
Reflex Signs. The finger flexor reflex (C8–T1) is depressed or absent.
Autonomic Signs. When the first thoracic root is injured, the sympathetic fibers, destined for the superior cervical ganglion (and eventually the eye, upper lid, and face), are interrupted. Therefore, an ipsilateral Horner syndrome (ptosis, miosis, and anhidrosis) results.
Lesions of the Cords of the Brachial Plexus
LESIONS OF THE LATERAL CORD
Lateral cord lesions are usually due to surgical or local trauma and result in paresis of the muscles innervated by the musculocutaneous nerve and the lateral head of the median nerve. Therefore, there is paresis of the biceps, brachialis, and coracobrachialis (which control elbow flexion and forearm supination) because of musculocutaneous nerve injury, as well as paresis of all muscles innervated by the median nerve except the intrinsic hand muscles. As a result, the following muscles are weak: pronator teres (forearm pronation), flexor carpi radialis (radial wrist flexion), palmaris longus (wrist flexion), flexor digitorum superficialis (middle phalangeal flexion of the second through fourth digits), flexor pollicis longus (flexion of the distal phalanges of the thumb), flexor digitorum profundus I and II (flexion of the distal phalanges of the second and third fingers), and pronator quadratus (forearm pronation). The biceps reflex is depressed or absent. Sensory loss may occur on the lateral forearm (the area of distribution of the lateral cutaneous nerve of the forearm, a branch of the musculocutaneous nerve).
LESIONS OF THE MEDIAL CORD
Lesions of the medial cord of the brachial plexus result in weakness of the muscles innervated by the ulnar nerve and the medial head of the median nerve (the median-innervated intrinsic hand muscles). The ulnar muscles involved are the flexor carpi ulnaris (ulnar wrist flexion), flexor digitorum III and IV (flexion of the terminal digits of the fourth and fifth fingers), and all the ulnar-innervated small hand muscles. The median muscles involved are the abductor pollicis brevis (abduction of the metacarpal of the thumb), opponens pollicis (opposition of the thumb), superficial head of the flexor pollicis brevis (flexion of the proximal phalanx of the thumb), and the first and second lumbricals. With proximal lesions of the medial cord, the medial anterior thoracic nerve may be injured, resulting in some paresis of the lower sternocostal portion of the pectoralis major muscle and of the pectoralis minor. The finger flexor reflex is decreased or absent. Because the medial cutaneous nerves of the arm and forearm are branches of the medial cord, a sensory loss may be evident on the medial arm and forearm.
LESIONS OF THE POSTERIOR CORD
Lesions of the posterior cord result in disability in the fields of distribution of the subscapular, thoracodorsal, axillary, and radial nerves. Subscapular nerve injury results in paresis of the teres major and subscapularis (internal rotators of the humerus), whereas thoracodorsal nerve injury results in latissimus dorsi paresis. Axillary injury manifests as deltoid (arm abduction) and teres minor (lateral rotation of the shoulder joint) paresis, as well as variable sensory loss in the distribution of the lateral cutaneous nerve of the arm (skin of the lateral arm). Radial injury results in paresis of elbow extension, wrist extension, forearm supination, and finger extension; there is a lesser degree of paresis of elbow flexion. When the radial nerve is involved, the triceps and radial reflexes are decreased or absent, and a variable sensory loss is present on the entire extensor surface of the arm and forearm and on the back of the hand and dorsum of the first four fingers.
Brachial Mononeuropathies
Injuries to individual peripheral nerves arising directly from the plexus are usually related to closed trauma (e.g., traction and compression injuries) or disease of the vasa nervorum (e.g., diabetic neuropathy). The clinical signs involve motor, reflex, and sensory disturbances in the entire distribution of each nerve involved. These findings are described in Chapter 2.
Thoracic Outlet Syndrome (Cervicobrachial Neurovascular Compression Syndrome)
The thoracic outlet syndrome results from compression of the brachial plexus or the subclavian vessels in the space between the first rib and the clavicle (thoracic outlet) [23,111,145].
There are usually various predisposing compressive factors, including a cervical rib, an enlarged seventh cervical transverse process, a hypertrophied anterior scalene muscle (scalenus anticus syndrome), clavicular abnormalities (congenital or traumatic), or a fibrous band uniting the seventh cervical transverse process to the first rib or anterior scalene muscle [23].
The thoracic outlet syndrome [23] may be purely vascular, purely neuropathic, or, rarely, mixed.
VASCULAR SIGNS AND SYMPTOMS
Vascular thoracic outlet syndrome may be arterial or venous. With subclavian artery compression there may be recurrent coldness, cyanosis, and pallor of the hand. Frank gangrene of the digits or Raynaud’s phenomenon is rare. A bruit may be present over the supra- or infraclavicular areas, especially when the arm is fully abducted. When the arm is abducted to 90 degrees and externally rotated, the radial pulse is frequently obliterated; however, pulse obliteration is occasionally seen in healthy individuals, and this maneuver is a poor diagnostic test for arterial compression [115]. The subclavian vein may also be compressed, resulting in arm edema, cyanosis, and prominence of the veins of the arm and chest.
NEUROPATHIC SIGNS AND SYMPTOMS
True neurogenic thoracic outlet syndrome is extremely rare [23,83,143,145,146], and occurs most frequently in young to middle-aged women. Usually, the lower trunk or medial cord of the brachial plexus is involved. Pain is the most common sensory symptom, is often intermittent, and is referred to the ulnar border of the hand and the medial forearm and arm [84]. Paresthesias and sensory loss may occur in the same distribution. The motor and reflex findings are essentially those of a lower plexus palsy. Involvement of the lower trunk may be restricted to those fibers derived from the eighth cervical root; therefore, thenar wasting and paresis (median innervation) may be prominent, whereas ulnar-supplied muscles are spared (the ulnar hand muscles derive innervation from the C8 and T1 roots, but the median thenar muscles are predominantly innervated by the C8 root) [83]. Upper plexus thoracic outlet syndrome may occur rarely [92].
A droopy shoulder syndrome has been described in patients with thoracic outlet syndrome [124]. This syndrome consists of the following signs and symptoms:
1. Low-set, droopy shoulders, and a long swan neck with horizontal or downsloping clavicles
2. Pain or paresthesias in the neck, shoulder, chest, arms, or hands
3. Aggravation of symptoms by downward traction and relief by propping up the arms
4. Occurrence predominantly in women
5. Absence of vascular, neurologic, and electrophysiologic abnormalities
6. A Tinel’s sign over the brachial plexus
7. The second thoracic vertebra visible above the shoulder on lateral cervical spine films
The Lumbosacral Plexus
Anatomy
The lumbosacral plexus (Fig. 3.4) derives from the ventral primary rami of the twelfth thoracic through fourth sacral levels and is situated within the substance of the psoas major muscle. Anomalous derivations of the plexus (prefixed or postfixed) occur in up to 20% of healthy subjects. The lumbosacral plexus gives off the following nerves (the distribution areas of these nerves are discussed in Chapter 2):
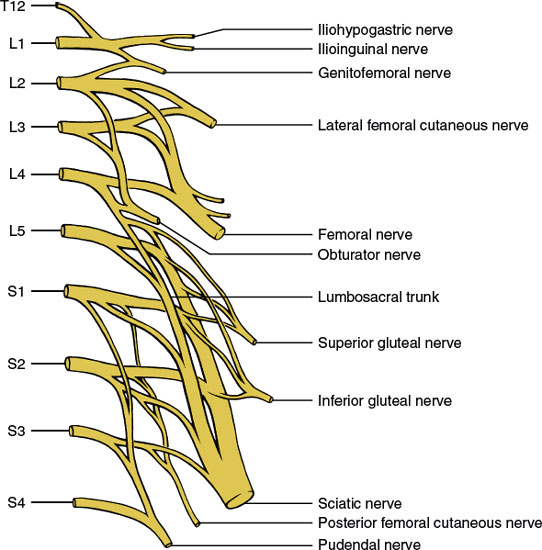
FIG. 3.4. The lumbosacral plexus.









