36 Cervical Laminoplasty
I. Key Points
– Laminoplasty is a procedure in which the spinal canal is expanded with the integrity of the posterior elements preserved.
– Laminoplasty was developed in Japan in the 1970s as a “tissue-sparing” alternative to laminectomy to minimize its associated risk of instability and progressive kyphotic deformity and thus improve outcomes.1
II. Indications
– Multilevel spinal cord compression secondary to one or any combination of the following:
• Ossification of the posterior longitudinal ligament (OPLL)
• Cervical spondylosis
• Congenital canal stenosis
– For consideration in children requiring laminectomies
– Not for isolated neck pain or for patients with a significant kyphotic deformity
III. Technique
– In cases of severe stenosis, awake fiberoptic intubation should be considered to minimize the risk of hyperextension injury and allow for reexamination of patient prior to positioning.
– Place neurophysiologic monitoring leads (include somatosensory evoked potentials [SSEPs], motor evoked potentials [MEPs], and electromyograms [EMGs]) and obtain baseline recordings prior to turning the patient.
– Secure patient’s head with or without pins (e.g., Mayfield three-pin headrest [Schaerer Mayfield, Randolph, MA]) and turn the patient prone with the head secured in a neutral to slightly flexed position.
– Tape superior and dorsolateral aspects of both shoulders and secure to the caudal corner of the operating table to aid in radiographic visualization of the lower cervical vertebrae.
– Use fluoroscopy to plan an incision extending from the spinous process of C2 to that of T1 and infiltrate with 1% lidocaine with epinephrine to minimize skin bleeding.
– Make a midline incision and use a subperiosteal dissection to reflect the paraspinal muscles from the caudal end of C2 to the rostral limit of T1.
– Remove the caudal third of the C2 lamina and the rostral third of the T1 lamina using the combination of a high-speed air drill and a 2 mm Kerrison punch, allowing visualization of the underlying dura at these levels.
– Remove the spinous processes from C3 to C7 with a rongeur and morselize the bone for subsequent autografting.
– Perform laminoplasty of lamina C3 to C7 by creating an “open” side and a “hinged” side. The open side is generally the side with the greatest compression and/or the side most clinically symptomatic.
• Use the high-speed air drill with a small drill bit to create troughs at the level of the lamina-facet junction from C3 to C7 (Fig. 36.1).
• Drill through the outer and inner cortical margins of the lamina on the open side, but only through the outer cortical margin and cancellous bone (and not the inner cortex) on the hinged side.
– Prepare the bone allografts to stabilize the canal expansion.
• Using rib allografts and the high-speed drill, cut three separate grafts each about 15 to 18 mm in length.
• Make transverse grooves along the cut surfaces of the rib grafts, approximating the thickness of the cut laminae.
– Prepare to “open the door.”
• Use two small curettes placed into the trough on the open side just deep to the outer cortex.
• Pull the curettes upward, thereby enlarging the lamina-facet gap and creating a green-stick fracture along the trough on the hinged side. Repeat the process on each successive lamina to expand the canal by about 4 mm (Fig. 36.2).
– Place the rib allografts in the gaps that have been created at the C3, C5, and C7 levels (Fig. 36.3).
– Place the morselized spinous process autograft over the decorticated bone surfaces of the facet and lamina on the hinged side at C3, C5, and C7 to provide for an intersegmental fusion.
– If the patient suffers from radiculopathy as well as myelopathy, one or more foraminotomies can be performed:
• Once the lamina has been elevated and the ligamentum flavum excised, drill the mesial third of the facet over the exiting nerve root and widen the opening as needed with 1 or 2 mm angled Kerrison punches.
– Leave the subfascial drain (e.g., Hemovac [Gohar Shafa, Tehran, Iran]) in situ to minimize hematoma formation.
– Variations in the approach
• Use spinous processes as autograft instead of the rib allograft
• Stabilize the rib allograft with mini-plates to the adjacent lamina and facet on the open side or sutures
• Use titanium spacers to hold open lamina that is affixed to the lamina-facet joint on the open side
• Perform full-thickness splitting of the lamina in the midline and create bilateral troughs, and then spread the lamina and place allograft spacers (double-door laminoplasty)
• Should rigid stabilization be required, lateral mass screws can be placed. This is best done after drilling and “opening the door,” but prior to graft insertion.
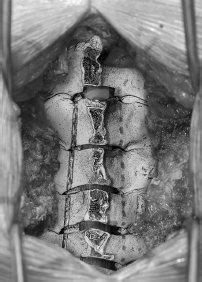
Fig. 36.1 Trough is drilled through the inner and outer cortices at the laminafacet junction on the left (shown from C3 to C6 only). Partial laminectomy of C2 is shown. Purple crosshatching denotes area of drilling for closed door side.
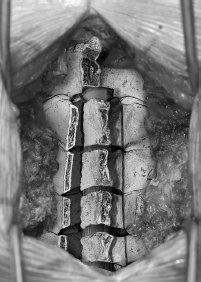
Fig. 36.2 With a trough drilled through the outer cortex only on the contralateral side (closed door side), the laminectomy door is “opened,” creating a greensticktype fracture on the closed side.

Fig. 36.3 Rib allograft is cut and secured into opening in lamina to expand the spinal canal, typically at C3, C5, and C7.
IV. Complications
Early
– Wound infection (2%)
– Dural tear/CSF leak (<1%)
– Hemorrhage (<1%)
– Spinal cord injury (<1%)
– Nerve root injury
– Delayed C5 nerve root injury (2 to 13.3%)
Late
– Postoperative neck pain (40 to 60%)
– Reduced range of motion (20 to 50%)
– New-onset kyphosis (2 to 15%)
V. Postoperative Care
– Mobilize early with cervical bracing.
– Discharge to home once patient is ambulating and tolerating full diet—usually 2 to 3 days postoperatively.
VI. Outcomes
– Clinical outcome studies are drawn largely from the Japanese literature on patients with OPLL and are difficult to compare as a result of patient heterogeneity and different outcome measures used.2
– Long-term (5 to 10 years) outcome studies suggest 50 to 70% of patients with either OPLL or cervical spondylitic myelopathy show improvement in their neurologic function following laminoplasty.3
– Limited studies comparing this procedure to its corresponding anterior procedure identify similar clinical outcomes, but overall there is a suggestion that outcomes are better than with laminectomy alone.
– Recent studies suggest that clinical outcomes are similar between (1) laminectomy and fusion and (2) laminoplasty, with preserved range of motion and overall lower surgical costs for the latter.
VII Surgical Pearls
– Obtain pre-op x-rays to rule out instability or kyphotic deformity.
– Ensure the provision of drilling gutters at the lamina-facet junction (and not lateral to it) and direct drill medial so as not to disrupt the facet joint
– Lift gently when opening the door and ensure that drilling of the closed side (through outer cortex and some cancellous bone) is adequate to avoid creating a true fracture of the lamina. With a true greenstick fracture some tension should remain in the opening to help maintain compression on the rib graft to hold it in place.
– If multiple foraminotomies are required, plan on making the open side the side where more foraminotomies are required.
– Visually inspect the construct and gently stress it once rib allograft is placed to ensure that you have not compromised the canal and that it remains stable.
Common Clinical Questions
1. True or false: Patients with kyphotic deformities and symptoms of myelopathy may benefit from laminoplasty procedures.
2. List three perioperative surgical complications associated with laminoplasty.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







