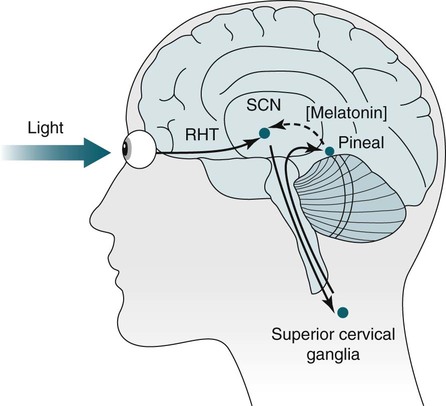
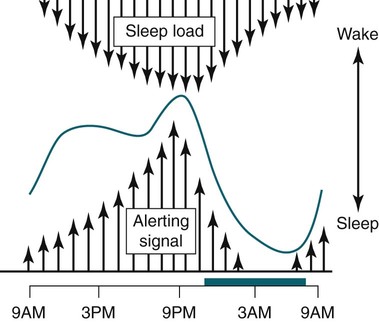
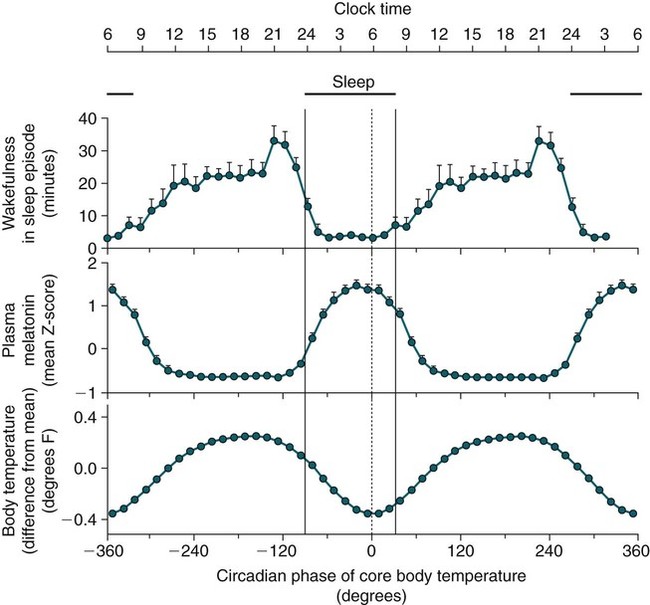
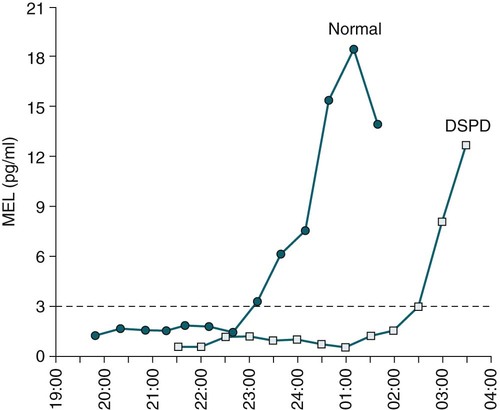
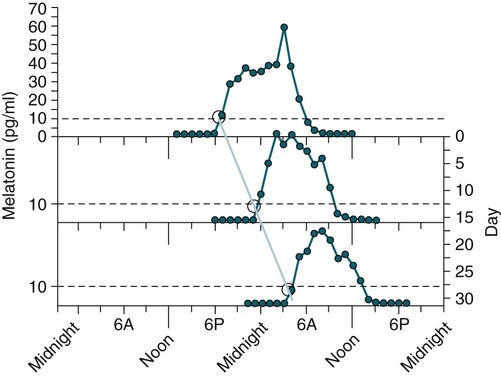
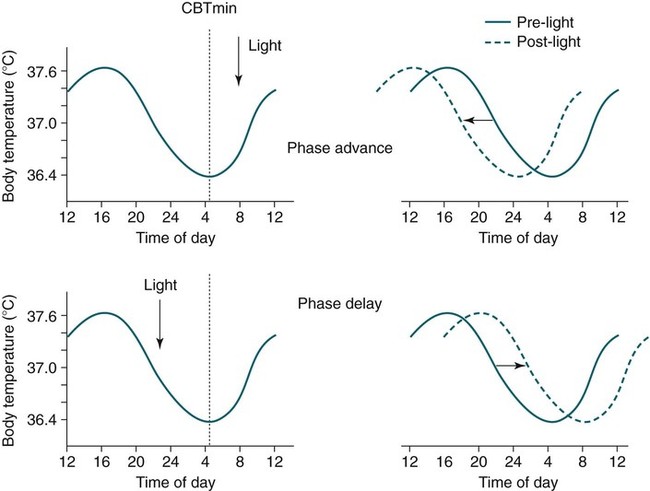
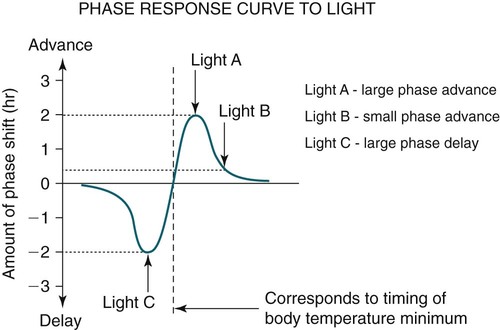
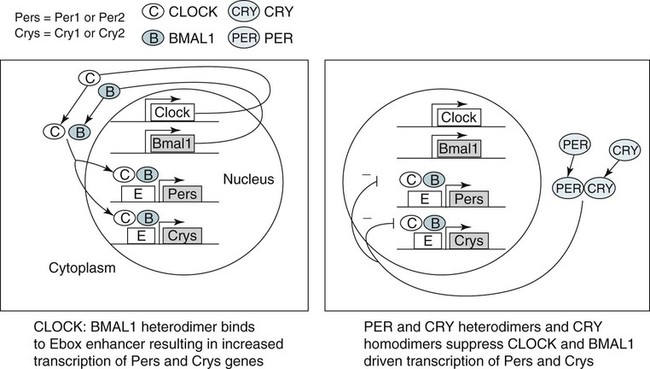
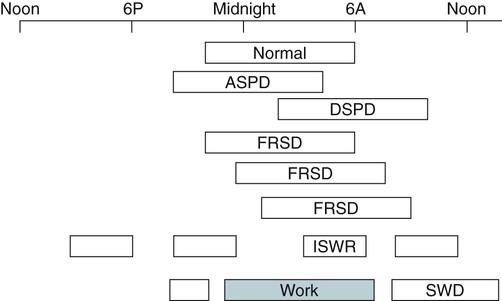
• In humans, the normal circadian period (tau) is approximately 24.2 hours (mean value). Because the period is slightly longer than 24 hours, this requires a slight phase advance daily to maintain entrainment with the light-dark cycle. • The major circadian pacemaker in humans is the SCN. The SCN is entrained to the light-dark cycle via light stimulation of retinal melanopsin-containing pRGCs. The ganglion cells communicate via the RHT to the SCN. Blue light has the most potent effect. • The alerting signal from the SCN increases during the day to counter the increasing homeostatic sleep drive (accumulated wake since the last sleep). The alerting signal falls during sleep and the homeostatic sleep drive also falls. • Melatonin is secreted in darkness by the pineal gland. Light inhibits melatonin secretion by decreasing the activating influences of neurons in the PVH nucleus. The neural pathway from the PVH neurons to the pineal gland is circuitous passing through the spinal cord and superior cervical ganglion (see Fig. 26–2). Melatonin binds to receptors on the SCN and decreases the alerting signal during darkness (promoting sleep). • The CBTmin occurs about 2 hours before the habitual wake time. • DLMO is the time that serum melatonin starts to increase above daytime levels under dim light conditions and occurs about 2 to 3 hours before bedtime or 7 hours before CBTmin. • CBTmin and DLMO can be used as markers of circadian phase. When the circadian rhythm of the body (CBTmin or DLMO) moves to a later clock time, this is said to represent a phase delay in circadian rhythms and to an early time phase advance. • Light after the CBTmin induces a phase advance and light before the CBTmin induces a phase delay. • Melatonin administered before the CBTmin induces a phase advance. Melatonin given after the CBTmin induces a phase delay. • Bright light “pushes” and melatonin “pulls” on the circadian phase (CBTmin) • The DSPD is characterized by a delay in sleep period relative to clock time, characterized by late sleep onset and late natural awakening. The DSPD is the most common CRSD seen in sleep clinics and presents as sleep-onset insomnia. If undisturbed by social obligations, patients with DSPD have good sleep maintenance and awaken feeling refreshed. • Treatments for the DSPD include bright light at or slightly before the natural (unrestricted) wake time (timed to follow CBTmin) or melatonin 5 to 7 hours before habitual sleep-onset time. Both melatonin and light can be used. The patient attempts to go to bed about • The ASPD is characterized by an advance in the sleep period relative to clock time characterized by early sleep onset and early awakening. It occurs most commonly in elderly individuals. • The CRSD-FRT is characterized by a progressive phase delay of 1 to 2 hours per day. It is most common in blind individuals but can occur in sighted individuals and has been described after head trauma. • The treatment for nonsighted patients with the CRSD-FRT is melatonin given about 1 hour before the desired bedtime. In some studies, a lower dose was more effective than a large dose. • The ISWR is characterized by at least three different periods of sleep during 24 hours (usually normal total sleep time). This CRSD is most common in elderly patients in nursing homes or institutionalized severely mentally impaired young patients. Treatments include bright light during the day, structured daily activities, and decreased noise and light at night. Melatonin at night is recommended for younger patients with mental retardation but not for elderly nursing home patients. • Approximately 10% of patients working a night shift have complaints of difficulty staying awake at night or sleeping during the day that are severe enough to be considered an SWD. • Treatment for night shift workers includes scheduled naps before the shift, bright light during the start of the shift, stimulants (caffeine or modafinil) at the start of the shift, dark glasses on the drive home (if after sunrise), and melatonin or hypnotics before the daytime sleep period. The major sleep period should occur soon after arriving home from work. The sleep environment should be as quiet and dark as possible. • Jet lag is characterized by a misalignment between endogenous circadian rhythms and local time caused by rapid travel across at least two time zones. Eastward travel requires adaptation by a phase advance and adaptation is more difficult than westward travel. Westward travel requires adaptation by a phase delay and adaptation is easier because of the intrinsic tendency for phase delay. Trying to avoid light at times inducing the wrong phase shift and receiving light exposure at correct times inducing the desired phase shift at the new destination are recommended. Melatonin before the desired sleep period or hypnotics may be helpful. Most individuals require about 1 day of adaptation for each time zone crossed. The word circadian means “about a day” and describes processes that vary over time with approximately a 24-hour period. In humans, many physiologic processes vary periodically on a nearly 24-hour schedule.1,2 The major circadian pacemaker in mammals is the suprachiasmatic nucleus (SCN) in the anterior hypothalamus. The nucleus exists as paired structures on each side of the third ventricle above the optic chiasm.2–4 The SCN contains cells that oscillate independently with a period slightly longer than 24 hours. The SCN controls the rhythms of core body temperature and sleep-wake propensity as well as the secretion of certain hormones (melatonin and cortisol). The period of the rhythm is called tau and the mean value in humans is about 24.2 hours.3 Some individuals have a slightly shorter tau and some longer. For humans to maintain synchrony with the light-dark cycle, external stimuli must induce a slight daily advance (shift in circadian rhythms to an earlier clock time) to counteract the intrinsic tendency for phase delay due to a period slightly longer than 24 hours. These external stimuli called zeitgebers (“time givers”) are said to “entrain” the SCN to the light-dark cycle. The most potent zeitgeber is non–visual light information. Other zeitgebers include exercise, food, and social activities. The light stimulus reaches the SCN via the retinohypothalamic tract (RHT) (Fig. 26–1). The RHT is a monosynaptic pathway connecting the melanopsin-containing photosensitive retinal ganglion cells (pRGCs) to the SCN. Nonvisual photoreception also mediates the pupillary light response. Some blind patients continue to be entrained by light due to residual function of the retinal ganglion cells. Whereas the ganglion cells are the major circadian photosensors, the rods and cones also contribute some nonvisual information via communication with the pRGCs.4,5 The shorter wavelengths of light (blue) have the greatest effect on circadian rhythms. The primary neurotransmitter of the retinal ganglion cell neurons in the RHT is glutamate. However, the neurons also release pituitary adenyl cyclase–activating peptide (PACP) as a co-transmitter that causes similar effects on the SCN neurons. The effects of glutamate are mediated by binding to N-methyl-d-aspartate (NMDA)-type glutamate receptors and the subsequent elevations of intracellular calcium and nitric oxide in the neurons of the SCN. PACP-containing fibers from the retinal ganglion cells also project to the intergeniculate leaflet (IGL), which in turn, project to the SCN. Neurons in the IGL use gamma-aminobutyric acid (GABA) and neuropeptide Y as co-transmitters. Neurons in the IGL may mediate some of the phase-shifting influences of exercise on the SCN. As is discussed later, the intensity, the duration, and the timing of light exposure determine the effect of light on the circadian system. The pineal gland secretes a hormone called melatonin during the dark cycle.4–11 In the absence of light, certain dorsal parvocellular neurons in the autonomic subdivision of the paraventricular hypothalamic nucleus (PVH) provide tonic stimulation to the pineal gland via a circuitous pathway4–7 (Fig. 26–2). These PVH glutaminergic neurons project to sympathetic preganglionic neurons in the intermediolateral cell column (IML) of the upper thoracic spinal cord. The preganglionic sympathetic neurons provide a cholinergic projection to postganglionic neurons located in the superior cervical ganglion. The postganglionic neurons are noradrenergic and project to the pineal gland. The release of norepinephrine stimulates the pineal gland via alpha and beta receptors (mainly beta 1). Noradrenergic stimulation on the pineal gland results in increased cyclic adenosine monophosphate (AMP) in the pinealocytes and this induces expression of serotonin N-acetyltransferase (also known as arylalkylamine N-acetyltransferase [AA-NAT]). This enzyme catalyzes the rate-limiting step in the synthesis of melatonin. Therefore, the amount of this enzyme controls the production of melatonin. In the presence of light, some neurons of the SCN directly inhibit those neurons in the PVH that are responsible for stimulating the pineal gland to secrete melatonin. Thus, light inhibits melatonin secretion and the absence of inhibition (absence of light) allows secretion of melatonin. Melatonin is sometimes called the dark hormone. The melatonin secreted by the pineal gland provides inhibitory feedback information to SCN neurons. Therefore, the SCN and pineal gland are mutually inhibitory. Important facts about human circadian rhythms are summarized in Box 26–1. Melatonin is not essential for circadian rhythms in humans because removal of the pineal gland has minimal effects. In other species such as birds, the pineal gland is essential. The SCN has a high density of two types of melatonin receptors (MT1 and MT2). The MT1 receptor is a G protein–coupled receptor that activates protein kinase C. When melatonin binds the MT1 receptor on SCN neurons, this decreases SCN-alerting signal. The MT2 receptor is a G protein–coupled receptor that inhibits the guanine cyclase pathway and results in a shift in circadian phase. A third type of melatonin receptor (MT3) does not affect the pineal gland. Exogenous melatonin by oral administration can also affect the SCN. The half-life of exogenous melatonin is short (30–45 min) unless sustained-release melatonin preparations are used. As might be expected, the effects of exogenous melatonin are largest when no endogenous melatonin is being secreted.8 Exogenous melatonin can decrease the SCN-alerting signal (hypnotic effects) and cause a phase shift of circadian rhythms (discussed in a later section). Melatonin acting on blood vessels in the skin causes vasodilatation and increased blood flow results in heat loss and lowering of the body temperature. The SCN helps maintain alertness by producing an alerting signal during the day and helps maintains sleep by producing a reduced signal at night. The other major influence on sleep propensity is the amount of accumulated wakefulness (time since the last sleep). This homeostatic process (sleep load) builds during wakefulness and then falls during sleep. As the pressure for sleep builds, the circadian signal increases to help maintain alertness despite a growing sleep debt but then decreases so that sleep can occur (Fig. 26–3). The two-process model considers the interaction of process S (homeostatic sleep drive) and process C (circadian rhythms, driven in large part by the SCN). The opponent model of sleep2,9 (SCN-alerting signal vs. sleep load) is illustrated in Figure 26–3. Alertness peaks during the early evening hours. There is a midday decrease in alertness around 2 to 4 pm and the lowest alertness is 4 to 6 am. The interaction of the opponent processes (homeostatic sleep load and circadian alerting signal) allows humans to be alert during the day and to sleep at night (Table 26–1). The secretion of melatonin at night exerts an inhibitory influence on the SCN that helps maintain sleep by reducing the alerting signal. TABLE 26–1 The pathways by which the alerting signal of the SCN regulates sleep-wake are complex.2,5 One of the major pathways is as follows. Neurons in the SCN project to neurons in the ventral subparaventricular zone (vSPZ). This area is immediately dorsal to the SCN. Neurons in the vSPZ then project to the dorsal medial hypothalamus (DMH). Glutaminergic neurons in the DMH project to the lateral hypothalamus neurons producing hypocretin (stabilizing wake-to-sleep transitions). In addition, DMH neurons using GABA as an inhibitory neurotransmitter project to the ventrolateral preoptic area (VLPO), a sleep-promoting area. These pathways mediate some of the effect of the SCN-alerting signal by inhibiting sleep-promoting areas and stimulating areas stabilizing transitions from wake to sleep. The minimum of the core body temperature (CBTmin) and the dim light melatonin onset (DLMO) are two useful markers of the position of an individual’s circadian rhythms with respect to the external environment (i.e., time of day) (Box 26–2). The CBTmin occurs about 2 hours before spontaneous awakening from nocturnal sleep (4–5 am in most individuals) (Fig. 26–4).10–12 The reduction in core body temperature during the sleep period corresponds to the elevation in plasma melatonin. The wakefulness in sleep episode is an estimate of the wake propensity and is maximum in the evening before the sleep period and falls during sleep. The DLMO occurs about 2 to 3 hours before typical bedtime.10–15 One can estimate the timing of the CBTmin as DLMO + 7 hours (see Box 26–2). The DLMO is determined by interval measurement of salivary or plasma melatonin performed in dim light (5 lux; because light inhibits melatonin secretion) in the evening. A rise in melatonin levels detects the DLMO time (Fig. 26–5). The melatonin midpoint can also be used as a circadian marker and occurs about 2 hours before CBTmin. When the circadian rhythm of the body (CBTmin or DLMO) moves to a later clock time, this is said to represent a phase delay in circadian rhythms and to an early time phase advance. The relationship between the timing of sleep and the circadian phase (as estimated by a circadian marker) can be quantified by the time interval (phase angle) between the two rhythms. In Figure 26–6, the progressive delay in the DLMO is evidence of a progressive delay in circadian rhythm in a patient with the circadian rhythm sleep disorder—free-running type (CRSD-FRT), also called the free-running disorder (FRD).14 Use of the core body temperature as a marker of circadian rhythms is complicated by the fact that eating, activity, and sleep can affect the timing of CBTmin. In research settings, a constant routine protocol is utilized in which the subject is kept awake at bedrest and fed equally distributed small meals for at least 24 hours. An alternative to the constant routine protocol is to use mathematical adjustments to the temperature rhythm. The DLMO can be affected (masked) by posture and drugs such as beta blockers and caffeine. One can monitor plasma melatonin (threshold > 10 pg/mL), salivary melatonin (>3 pg/mL), or urinary melatonin metabolite (6-sulfatoxymelatonin [aMT6s]) (see Fig. 26–6). Exposure to light before the CBTmin causes a phase delay and light exposure after the CBTmin causes a phase advance (Fig. 26–7) in circadian rhythm.15–18 Thus, normal light exposure during the early morning induces a daily phase advance in the circadian rhythms to compensate for the intrinsic tendency to phase delay because tau is slightly longer than 24 hours. The amount of circadian rhythm shifting (also called phase change) depends on the timing of light as well as the intensity and duration of light (Box 26–3). In addition, the effect depends on the previous exposure to light.15–18 For example, a low intensity of light may cause significant shifting of the circadian rhythms in a patient staying in a dark room for several days. Light intensity is measured in lux. Indoor light is typically around 250 lux and outdoor bright light has an intensity over 100,000 lux. For humans exposed to outside light daily (>10,000 lux) for a portion of each day, a relatively high light intensity is needed to shift circadian rhythms. Outside daylight is much more effective at shifting the circadian phase than indoor light. When outdoor light exposure is not practical or possible, light boxes are available (2500 lux) for therapeutic phase shifting by light. One study of subjects kept in a dimly lighted environment found that increasing light intensity above 550 lux added relatively little to the phase shifting ability of light or the ability to suppress melatonin.19,20 A sigmoid-shaped relationship was noted (Fig. 26–8). However, for patients chronically exposed to much brighter light, a higher intensity of light would be needed for maximal effect. Natural light is composed of a spectrum from 380 nm (violet) to 760 (red). As noted previously, blue light (460 nm) has greater phase shifting properties than the rest of the visible light spectrum.20 This may be due to the properties of the melanopsin pigment that has maximum absorbance in this range. Recently, light boxes have become available with enriched blue light to minimize the intensity or duration needed for a therapeutic response to light therapy. However, a recent study showed no benefit of light enriched with blue light compared with white light.21 It is possible that new light boxes with light-emitting diode (LED) emission of monochromatic blue light could be more effective. Intermittent as well as continuous light is also effective at resetting the circadian pacemaker22,23 (Fig. 26–9). This fact has clinical implications for delivery light treatment when a patient cannot sit in front of a light box for long periods of time. The relationship between the timing of light exposure and the amount of phase shift is best presented using a phase response curve (PRC). The curves are constructed by plotting the amount of phase shift versus the timing of the light stimulus (constant stimulus intensity). By convention, the positive vertical axis represents phase advances and the negative axis phase delays. For light, the magnitude of phase shifting depends on the proximity to the CBTmin (Fig. 26–10). In most studies, the maximum phase shift occurs approximately 3 to 4 hours before (phase delay) or after (phase advance) the CBTmin (Fig. 26–11). Light in the middle of the day has minimal phase shifting effects. Figure 26–11 illustrates a PRC for light. This was determined using a constant routine, and shifts in the midpoint of melatonin secretion (melatonin midpoint) rather than DLMO were measured following a single pulse of light. Again, note that the biggest phase shifts occur when light is administered near the CBTmin. Relatively small doses of exogenous melatonin (0.3–0.5 mg) can shift the circadian phase (Table 26–2) if taken at the correct times. As might be expected, the phase shifting effects of exogenous melatonin are minimal during the dark period when the endogenous plasma level of melatonin is high.24–26 Melatonin in higher doses (3–5 mg) has a direct hypnotic effect8 as well as a chronobiotic effect. However, the hypnotic effects of melatonin are limited by the drug’s short half-life and the fact that if taken at night, the endogenous plasma melatonin is already high. TABLE 26–2 Phase Shifting with Melatonin and Hypnotic Effects • PRC approximately opposite to light PRC (12 hr out of phase). • Reversal point (phase advance to phase delay) may be slightly before minimum of core body temperature but is always considerably after the DLMO. • Dose-response curves may vary with dose (0.3 mg vs. 3 mg) • Note at larger doses, hypnotic effects are noted. • Exogenous melatonin half-life is about 30 to 45 minutes. • Given closer to bedtime, melatonin may reduce SCN alerting signal (dampen wake maintenance zone) • Maximal phase advance for different melatonin doses* *From Eastman CI, Burgess HJ: How to travel the world without jet lag. Sleep Med Clin 2009;4:241–255. The PRC curve for melatonin is roughly 12 hours opposite (out of phase) to the light PRC24–26 (Fig. 26–12). In displays of the melatonin PRC, the timing of melatonin is often expressed relative to DLMO but can also be expressed relative to the estimated CBTmin. Melatonin when given in the early evening before the DLMO results in a phase advance. Melatonin given at the end of the subjective night–early subjective day causes a phase delay (see Fig. 26–12). As expected, the melatonin PRC has flat region (no phase shifting) between the DLMO and the CBTmin (endogenous melatonin already high). The cross-over point for melatonin (transition from phase advance to phase delay) is during the night but may not precisely coincide with CBTmin. In addition, the shape of the melatonin PRC appears to depend on the dose of melatonin studied and the method of determination of the PRC (Fig. 26–13). Note that the timing of the maximum phase delay induced by melatonin is several hours after the CBTmin. Thus, taking melatonin 1 or 2 hours after spontaneous awakening causes the most phase delay. However, melatonin administration is often not used in the early morning because any hypnotic effects would not be well tolerated outside of a research setting. An exception is when morning sleep is desired by a person working a night shift. A summary of the effects of bright light and melatonin is presented in Figure 26–14 with illustrations of the use of these interventions in two circadian rhythm sleep disorders (CRSDs). Bright light in the evening causes a phase delay and in the early morning a phase advance. Melatonin in the early evening causes a phase advance and in the early morning a phase delay. A simple description of the effects of light and melatonin is that “bright light pushes and melatonin pulls the circadian rhythms.” In this figure, it is assumed that the CBTmin lies within the initial sleep period of the advanced sleep phase disorder (ASPD) and the delayed sleep phase disorder (DSPD) patients. The intrinsic 24-hour rhythm in the SCN neurons is due to the interactions of a number of genes.27,28 These genes form autoregulatory feedback loops on transcription (DNA to mRNA) and translation (mRNA to proteins) that drive the cycling pattern. The feedback is provided by the protein translational products that can either stimulate or repress further gene transcription. A brief description of a few of the many molecular mechanisms responsible for the 24-hour cycle in transcription, translation, and posttranscriptional protein metabolism that drives the rhythmic cycle of the circadian clock genes follows (Fig. 26–15 and Table 26–3). By convention, small letters refer to genes and capital letters refer to protein translational products. For example, transcription and translation of clock results in production of the protein CLOCK. The human forms of genes are denoted with a preceding h (e.g., hper2). TABLE 26–3 The International Classification of Sleep Disorders, second edition (ICSD-2), lists criteria for a CRSD (Box 26–4).1 There must be alteration of the circadian timing system, usually a misalignment between endogenous circadian rhythm and exogenous factors, that affects the timing or duration of sleep. The alteration in circadian timing must lead to a sleep complaint (insomnia, excessive sleepiness, or both). Finally, the change in wake-sleep must be associated with impairment including social or occupation functioning. For example, if a person’s normal sleep phase is quite delayed but is something that they desire, this would not be a CRSD.1 The ICSD-2 lists nine CRSDs (Box 26–5). A large proportion of normal individuals experience problems with sleep or alertness secondary to shift work or jet lag at some point in their lives. The boundary between what constitutes a normal response and what constitutes a disorder is blurry in some disorders such as the shift-work disorder. A schematic of the changes in the habitual sleep period is illustrated in Figure 26–16. Comprehensive reviews of the existing evidence for evaluation and treatment of these disorders and practice parameters for evaluation and treatment of CRSD were published by the American Academy of Sleep Medicine (AASM) in 2007.29–31 In evaluating patients for suspected CRSD, the physician utilizes history, a sleep log for at least 7 days, and often actigraphy (Box 26–6). The morningness-eveningness questionnaire (MEQ) is discussed in the following section. The MEQ and markers of circadian phase (DLMO) are used for research studies but the clinical utility for routine evaluation remains to be documented. Polysomnography is not indicated for evaluation of patients with CRSD unless another sleep disorder such as sleep apnea is suspected. The MEQ was developed by Horne and Ostberg in 1976.32 The MEQ contains 19 questions aimed at determining the natural propensity to perform certain activities during the daily temporal span. Most questions are framed in a preferential manner and require a response to specific times that an individual would prefer to do a certain activity (as opposed to when they actually do it). Each question has answers 0 to 6. The sum ranges from 16 to 86. Lower values correspond to evening types (see Box 26–6). The AASM practice parameters state that sleep logs and actigraphy are indicated for evaluation of patients with suspected or known CRSD31 (see Box 26–6). Figure 26–16 is a schematic diagram of changes in the sleep period with the different CRSDs compared with normal. In general, sleep logs or actigraphy documents a habitual sleep period compared with normal that is advanced (ASPD), delayed (DSPD), progressively delayed (FRD), irregular (irregular sleep wake rhythm [ISWR]), or with a daytime major sleep episode (shift-work disorder). Patients with the DSPD complain of inability to fall asleep at a socially acceptable time—a type of sleep-onset insomnia. If allowed to maintain their own chosen schedule, they would usually sleep for a fairly normal duration and feel rested on arising.1,16,17,33 However, because of societal pressures, they must awaken earlier than desired and are often sleepy during the day. Therefore, they complain of difficulty waking up and daytime sleepiness (short sleep duration). In contrast to behaviorally induced sleep delay, these patients cannot fall asleep earlier unless very sleep-deprived. Practice parameters for the evaluation and treatment of DSPD have been published (Box 26–7 and Table 26–4).
Circadian Rhythm Sleep Disorders
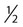 hour earlier each night and the timing of application of melatonin and light is changed to maintain the initial relationship.
hour earlier each night and the timing of application of melatonin and light is changed to maintain the initial relationship.
Melatonin
The SCN and Sleep-Wake Cycle
Nighttime sleep
First part of night
Second part of night
Daytime wakefulness
First part of day
Second part of day
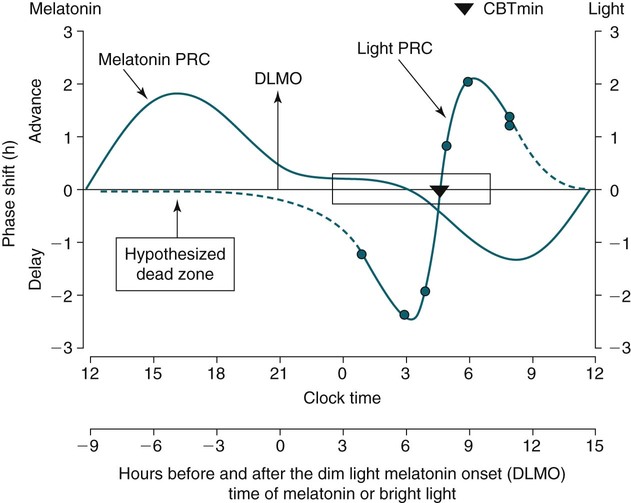
Markers of Circadian Phase
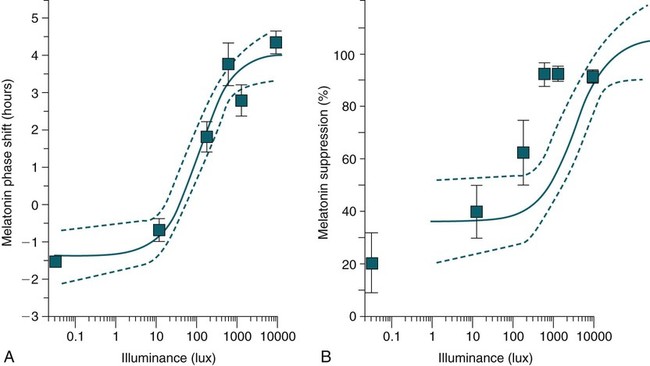
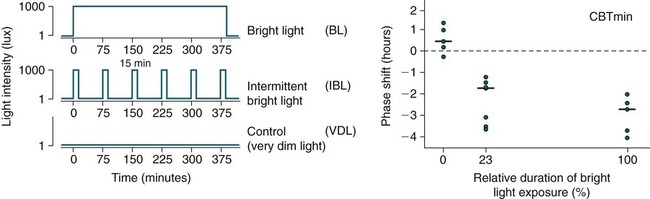
Shifting the Circadian Rhythms
Phase Shifting by Light
Phase-Response Curve for Light
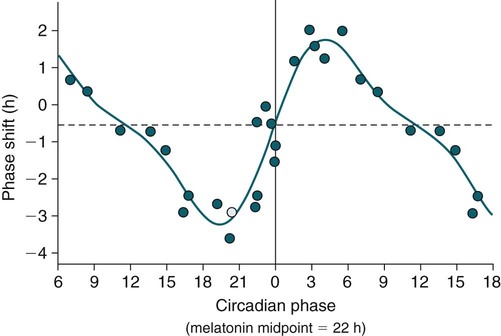
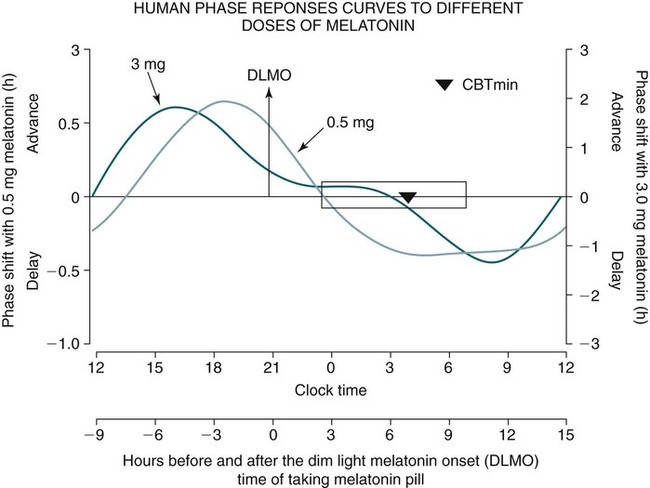
Phase Shifting by Exogenous Melatonin
Dose
0.3–0.5 mg
3 mg
OPTIMAL TIMING
Before DLMO
2–3 hr
5 hr
Before habitual sleep onset
4.5–5 hr
7.5 hr
Before CBTmin
9 hr
12 hr

Melatonin PRC Curve
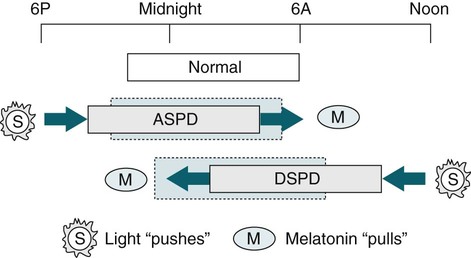
Summary of Effects of Light and Melatonin
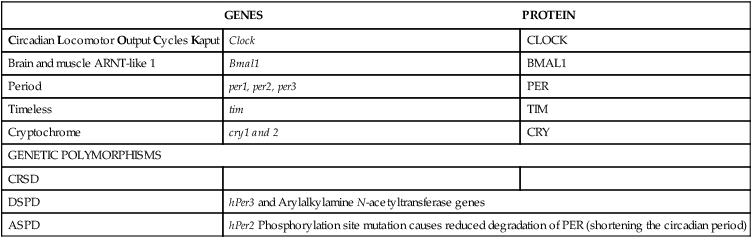
Genomics of CRSD
GENES
PROTEIN
Circadian Locomotor Output Cycles Kaput
Clock
CLOCK
Brain and muscle ARNT-like 1
Bmal1
BMAL1
Period
per1, per2, per3
PER
Timeless
tim
TIM
Cryptochrome
cry1 and 2
CRY
GENETIC POLYMORPHISMS
CRSD
DSPD
hPer3 and Arylalkylamine N-acetyltransferase genes
ASPD
hPer2 Phosphorylation site mutation causes reduced degradation of PER (shortening the circadian period)
Circadian Rhythm Sleep Disorders
Morningness-Eveningness Questionnaire
Sleep Logs and Actigraphy
Delayed Sleep Phase Disorder
Circadian Rhythm Sleep Disorders









