WHO class
Definition
Grade
Mitosis
Ki67%
I
NET
G1
<2
≤2
II
NET
G2
2–20
3–20
III
NEC
G3
>20
>20
IV
MANEC
nd
nd
nd
V
Hyperplasia/dysplasia
na
na
na
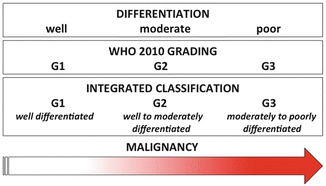
Fig. 6.1
Diagram illustrating the theoretical relationship existing between morphological differentiation, grading, and malignancy for neuroendocrine neoplasms. It is understood and accepted that the lower the degree of differentiation, the higher the malignancy is expected in a biological continuum. Grading, since based on unique and fixed descriptors, lies in between, and for G2 and G3, classes may well incorporate cases with different degree of differentiation along the very same continuum (see text)
Despite such limits, the current grading proved essentially effective for death prediction in multiple independent studies [15, 17, 25, 41, 42]. For pancreas, it was demonstrated that Ki67 5 % is the value above which the death risk increases in a statistically significant fashion as previously suggested [15, 25]. This value needs to be taken into adequate account for any specific decision for NET G2 patients.
G1-G2 NETs may display most types of the structures described originally by Soga and Tazawa in 1971 for carcinoids [43], namely, solid islet (type A), trabecular (type B), and glandular (type C) (Figs. 6.2 and 6.3). Usually, a pancreatic NET may display a mixture of types. The margins are usually expansive, with a delicate stroma rich in vessels, a feature typical of neuroendocrine cancer. Often, the stroma may become more solid with desmoplastic, parenchyma-substituting aspects reminiscent of the more aggressive adenocarcinoma. This latter feature may associate with deposits of amorphous eosinophilic material defined as amyloid and displaying metachromasia at Congo red stain. Amyloid deposits have been classically described in insulinomas and are composed by fragments of the hormonal peptide islet amyloid polypeptide (IAPP) as produced by the tumor beta cells [44]. NET cells are usually rather monomorphic, with abundant eosinophilic cytoplasm, round, monomorphic nuclei with spotted chromatin and rather rare mitoses, only exceptionally atypical. The degree of atypia does however increase in G2 cases, with prevalent solid structure, polymorphic cytological features including irregular cell size, pronounced atypia with increased nuclear/cytoplasmic ratio, evident nucleoli, and mitoses, sometimes atypical. Necrosis is absent and when present usually heralds the more severe cytological-histological features of NEC (Fig. 6.4). Angioinvasion and perineural space invasion may also be observed and, though of potential prognostic significance, are not included in the current classification criteria.
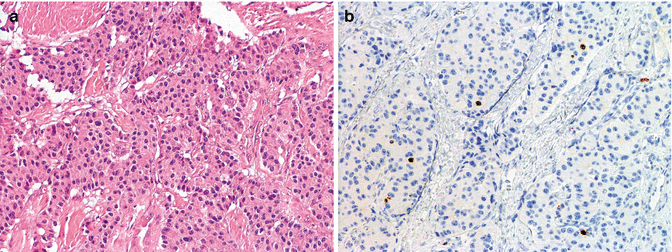
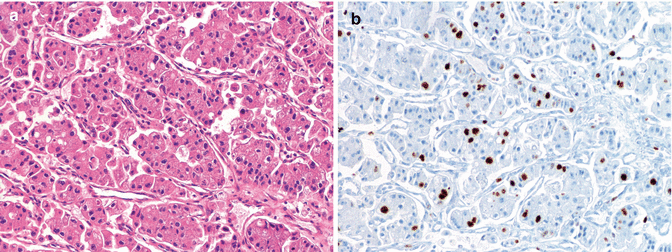
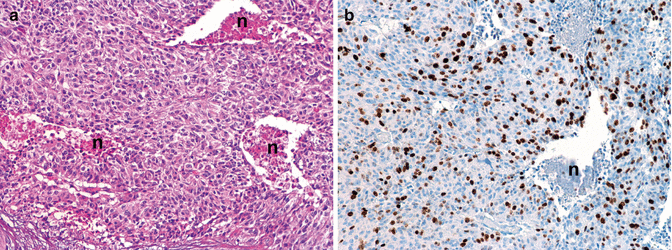

Fig. 6.2
Morphology of a PanNET G1 (WHO 2010) showing a mixed structure with trabeculae and solid islets, evident hyalinized stroma, mild atypia in absence of necrosis (a), and very few cells labeled for Ki67 corresponding to about 1 % (b); this case was a functioning, insulin-producing PanNET (insulinoma; see also Figs. 6.5 and 6.6) of 2 cm in size void of local lymph nodes and distant metastases (pT1 pN0 pM0, AJCC 2010; pT2 pN0 pM0 ENETS staging). (a) H & E (b) immunoperoxidase

Fig. 6.3
Morphology of a PanNET G2 (WHO 2010) showing a relatively more compact, trabecular structure with highly vascularized stroma, mild to moderate atypia with irregularly shaped nuclei (a) several of which are labeled for Ki67 and accounting for about 16 % (b); occasional foci of necrosis were also observed as well as 5 mitoses per 10 HPF (not shown); the case was a nonfunctioning PanNET of 2.7 cm in size, void of local lymph nodes and distant metastases (pT2 pN0 pM0, AJCC 2010, and ENETS staging). (a) H & E (b) immunoperoxidase

Fig. 6.4
Morphology of a high-grade neuroendocrine carcinoma, G3 NEC (WHO 2010) showing a packed trabecular-solid structure with multiple foci of necrosis (n), moderate atypia with irregular though monomorphic nuclei (a), and high Ki67 index accounting for about 40 % (b); the case was 2.5 cm in size with synchronous liver metastases in the absence of local lymph node deposits (pT2 pN0 pM1 AJCC 2010 and ENETS staging). (a) H & E (b) immunoperoxidase
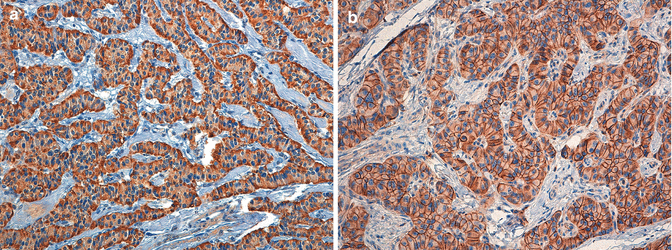
G1-G2 NETs stain positive for all markers of neuroendocrine differentiation, including chromogranin A and synaptophysin, the two minimum stains recommended for diagnosis by ENETS (Fig. 6.5) [16]. It is usually considered good practice that at least two neuroendocrine- specific stains should be positive in the large majority of tumor cells to set the diagnosis of NET. The degree of specificity of neuroendocrine stains is the highest for chromogranin A and decreases for synaptophysin, NSE, PGP 9.5, and CD56 N-CAM, all of which may stain non-neuroendocrine neoplasms. By converse, the search for cell-type-specific markers, namely, the peptide hormones like insulin, glucagon, pancreatic polypeptide, somatostatin, and ghrelin, is not required. When performed, usually it may show only a variable fraction of positive NET cells or, more rarely, a diffuse pattern of expression (Fig. 6.6a) [6]. The search for specific hormones is not mandatory for the diagnosis of NET but may be required for confirmation of a clinically functional condition. Other immunohistochemical stains may be required to answer specific clinical query or in the metastatic setting. In particular, the assessment of the somatostatin receptor subtype 2A (SSTR2A) may be requested on tissue when the in vivo assessment of lesions expressing the somatostatin receptors by nuclear medicine methods (e.g., Octreoscan® or Ga68-DOTA-NOC/TOC) is not available (Fig. 6.6b). Other markers including the nuclear transcription factors like the insulin gene enhancer protein islet-1 (ISL-1), the homeobox CDX2, and the NK2 homeobox 1 (NKX2-1), also known as thyroid transcription factor 1 (TTF-1), may be useful to determine the origin of a neuroendocrine cancer metastasis of potential pancreatic origin. Their use and significance need to be carefully weighed according to their known tissue-specificity limits and to the clinical evidence of the specific case under study.
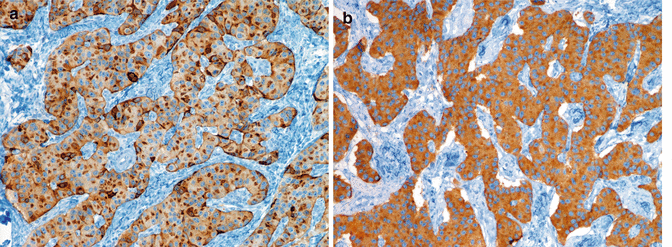

Fig. 6.5
Diffuse and intense expression of chromogranin A (a) and synaptophysin (b) in a functioning, insulin-producing PanNET G1 (insulinoma, same case of Figs. 6.2 and 6.6); note the irregular distribution of chromogranin A staining likely reflecting the chromogranin A-containing large dense-core vesicles (LDCV) as compared to the more uniform staining of synaptophysin (b). A and B immunoperoxidase
NECs by definition display a high proliferative fraction, though histology may reveal different features. A solid structure with some trabecular packed areas – a structure generally defined as “organoid” – is often observed, together with moderate to severe cytological atypia, sometimes with relatively abundant eosinophilic cytoplasm, polymorphic large nuclei, increased nuclear/cytoplasmic ratio, evident nucleoli, and multiple mitoses often atypical (Fig. 6.4). Such aspects may well refer to the “structures of lower or atypical differentiations” defined as Type D by Soga and Tazawa [43]. Necrosis in such cases is usually scant and spotty; however, it becomes more evident with increased large solid sheet structure and increased cell atypia with large nuclei and reduced cytoplasm, high-grade features more classically associated with large cell NECs. Not infrequently, cases showing areas with different histology of low and high grade may be observed [45]. In such cases, the highest-grade areas drive the diagnosis, according to the WHO 2010 grading rules. At immunohistochemistry, both chromogranin A and synaptophysin are widely expressed, though, while synaptophysin is usually intense, chromogranin A may be only scant and presenting as a thin rim of positive stain at cell periphery or as dots at Golgi cell site. Such features reflect the relative absence of large dense-core vesicles (LDCV) containing chromogranin A as observed at ultrastructure in NECs. Hormones in general are only rarely observed similar to SSTR2A, which however may be present in about 25–30 % of NECs [46]. Other neuroendocrine markers like NSE and CD56 N-CAM may be useful since they do not associate with neuroendocrine granules [13].
6.6 Pathological Classification: Staging
Staging is the second tool introduced by WHO 2010, following the indication by the International Union for Cancer Control (Union Internationale Contre le Cancer, UICC) and aligning with the American Joint Committee on Cancer (AJCC) of the American College of Surgeons (Table 6.2) [47, 48]. In the absence of any official staging for neuroendocrine neoplasms at any anatomical side, the staging schemes for the exocrine cancer counterparts were commonly utilized in clinical practice for neuroendocrine cancer patients. However, the biological difference between the two cancer types made such practice of limited clinical value. On such bases, ENETS proposed a neuroendocrine cancer-specific staging system for any gastroenteropancreatic anatomical site [27]. The ENETS scheme for pancreas neuroendocrine neoplasm proved effective in several studies [25, 41, 49–51]. The WHO/UICC/AJCC staging system adopted for the neuroendocrine neoplasms of the pancreas is however by definition the very same as for the adenocarcinoma. This generated some concern for its clinical application [52]. Both systems are indeed effective [17, 53, 54]; however, in a head to head comparison on a large cohort of about 1,000 patients, the ENETS system described more accurately the neuroendocrine cancer disease [15].
Table 6.2
Staging of pancreatic neuroendocrine neoplasms according to ENETS and UICC/AJCC/WHO 2010
ENETS | UICC/AJCC/WHO 2010 | ||||||
|---|---|---|---|---|---|---|---|
T definitions | |||||||
TX | Primary tumor cannot be assessed | Primary tumor cannot be assessed | |||||
T0 | No evidence of primary tumor | No evidence of primary tumor | |||||
Tis | na | Carcinoma in situ | |||||
T1 | Tumor limited to the pancreas and size <2 cm | Tumor limited to the pancreas, 2 cm or less in greatest dimension | |||||
T2 | Tumor limited to the pancreas and size 2–4 cm | Tumor limited to the pancreas, more than 2 cm in greatest dimension | |||||
T3 | Tumor limited to the pancreas and size >4 cm or invading duodenum or bile duct | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery | |||||
T4 | Tumor invading adjacent organs (stomach, spleen, colon, adrenal gland) or the wall of large vessels (celiac axis or the superior mesenteric artery) | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) | |||||
N definitions
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||||