Fig. 8.1
Large pancreatic insulinoma with amyloid stroma (hematoxylin eosin saffron, original magnification ×120)
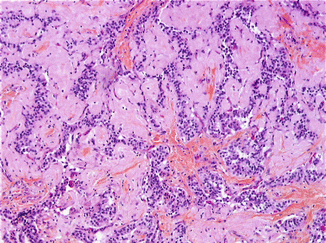
Fig. 8.2
Higher magnification showing well-differentiated neuroendocrine cells, without mitotic figures (hematoxylin eosin saffron, original magnification ×260)
Associated lesions should be looked for in surrounding tissue if the type of resection allows (a) other endocrine or non-endocrine tumors or (b) endocrine microadenomatosis, defined by an increase in the number and size of pancreatic insular structures, which retain diameters less than 5 mm. Such lesions might suggest a syndrome of familial predisposition to pancreatic endocrine tumor, especially the MEN-1 syndrome, but are not specific. In many cases, islet cell enlargement is only adaptive to repeated and/or prolonged insulin-induced hypoglycemias.
8.4.2 Immunohistochemistry
In almost all cases of insulinomas, it is possible to demonstrate the presence of insulin- and proinsulin-producing cells, but with a variable intensity of staining, from weak to strong. Some insulinomas do not react positively despite a correct diagnosis, likely as a result of a rapid release of the hormone from insulin-producing cells [1]. The subcellular distribution of hormonal products is variable [13]. In many cases, a normal pattern of intracellular distribution is retained: insulin is predominantly detected as granular deposits along the basal (secretory) pole of neoplastic cells, whereas proinsulin is predominantly detected in their perinuclear area. In other cases, insulin and proinsulin are diffusely detected all over the cytoplasm of neoplastic cells; this abnormal distribution pattern is associated with abnormalities in the proinsulin to insulin conversion. IAPP is also commonly detected in most insulinomas. It must be underlined that, in addition to insulin and proinsulin, it is not exceptional to detect other hormones in minor populations of neoplastic cells (Fig. 8.3).
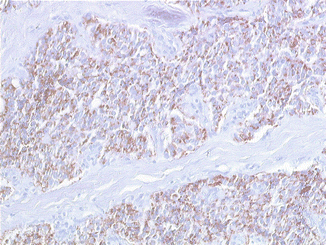

Fig. 8.3
Insulin expression with pericellular cytoplasmic distribution (indirect immunoperoxidase, original magnification ×260)
The demonstration of insulin or proinsulin in neoplastic cells is not absolutely required for a definitive diagnosis of insulinoma [1]. The main interest of insulin or proinsulin staining is to confirm the nature of a resected tumor, when multiple tumors are present in the same patient, or to confirm the nature of a possible metastatic deposit from a potentially malignant insulinoma.
The expression of several transcription factors associated with beta-cell lineage development, such as PDX1 and Islet-1, also termed ISL1, has been recently studied [14]. PDX1 is required for pancreatic development and beta-cell maturation; ISL1 is involved in endocrine islet embryogenesis and in beta-cell maturation. Insulinomas express only PDX1 and ISL1, but these two transcription factors can be expressed in all other pancreatic NET subsets, including gastrin-, glucagon-, and somatostatin-producing tumors. These markers are therefore a clue to the pancreatic origin of a neuroendocrine tumor, but their specificity is much lower than that of hormonal products for the identification of insulinomas in tissue sections.
It has been verified that insulinomas usually express, like other pancreatic NETs, several somatostatin receptors, including sst-2 and sst-5. However, somatostatin receptors are not detectable in no less than 30 % of insulinomas [15]: this proportion is high as compared to most other well- or very well-differentiated NETs. This is one of the reasons explaining the poor sensitivity of octreotide scintigraphy in this indication.
8.4.3 Genetics
Insulinomas are one of the most frequent tumor subset observed in the MEN-1 syndrome (Fig. 8.4). Rare cases of insulinomas have been also described in two other genetic predisposition syndromes, neurofibromatosis type 1 and tuberous sclerosis [1]. Von Hippel-Lindau (VHL) syndrome has not been described as being associated with insulinoma or with other forms of secreting NET.
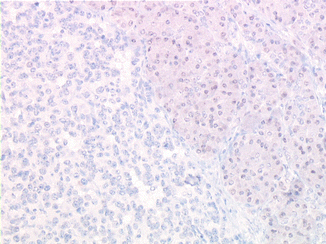

Fig. 8.4
Lack of detectable menin expression in insulinoma cells from a MEN-1 patient (indirect immunoperoxidase, original magnification ×350)
Despite the strong association between insulinomas and genetic predisposition syndromes, alterations in the corresponding genes are extremely rare in sporadic insulinomas: only a few cases have been reported in the literature. In the same way, genes frequently altered in sporadic nonfunctioning pancreatic NETs, such as MEN–1, DAXX, and ATRX, are only very rarely mutated in sporadic insulinomas. A recent study, using whole-exome sequencing, has pointed out to the possible involvement of YY1 gene in the pathogenesis of sporadic insulinomas [16]: T372R mutation of YY1 gene has been detected in 30 % of cases tested. YY1 encodes the Ying yang 1 protein involved in the regulation of mitochondrial function and insulin/IGF signaling within the pancreatic beta cell, in close interaction with the mTOR pathway. Further studies are required to confirm the degree of involvement of this gene and to identify other possible driver genes in sporadic insulinomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







