16 Clinical–Electrophysiologic Correlations
Overview and Common Patterns
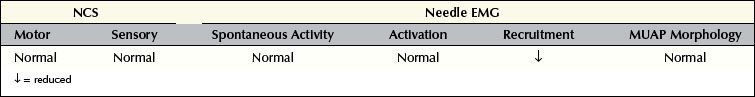
Neuropathic Lesions
Axonal Loss Lesions
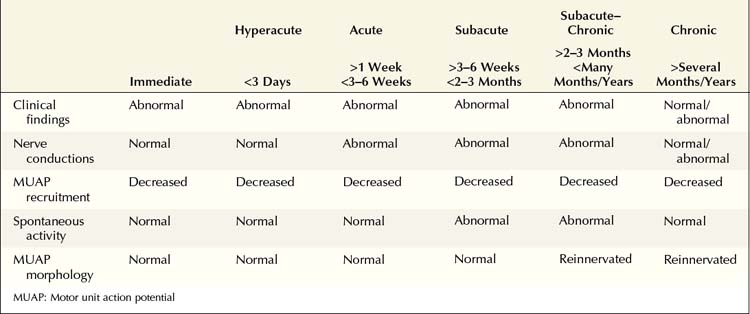
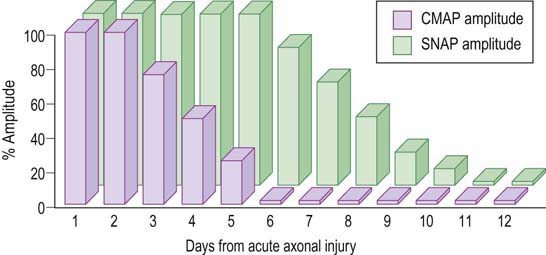
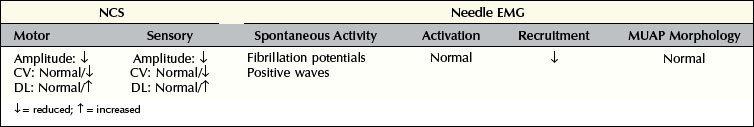
Understanding the pattern of changes that takes place over time (time-related changes) is essential in the interpretation of neuropathic lesions. With an axonal loss lesion, an orderly pattern of abnormalities develops over time on NCSs and EMG (Table 16–1). Immediately after an axonal loss lesion (e.g., partial transection of a nerve), clinical weakness and numbness develop. However, wallerian degeneration of the nerve does not occur until days 3 to 5 for motor fibers and days 6 to 10 for sensory fibers (Figure 16–1). Before that time, distal NCSs remain normal. Thus, when the nerve is both stimulated and recorded distal to the lesion, it can still conduct well despite being effectively disconnected from its proximal segment. After wallerian degeneration occurs, NCSs become abnormal, showing changes consistent with axonal loss: amplitudes decrease, with relative preservation of conduction velocities (CVs) and distal latencies (DLs). Amplitudes for motor studies decline slightly earlier than for sensory nerves; this likely occurs due to failure first at the NMJs. If the largest and fastest axons have also been lost, there may be some slowing of CV and DL, but never into the demyelinating range (i.e., CV <75% of lower limit of normal; DL >130% of upper limit of normal).
1. Lesion of the L5–S1 nerve roots (i.e., the longest distance between a lesion and the muscle). Fibrillation potentials and positive sharp waves take 10 to 14 days to develop in the paraspinal muscles, 2 to 3 weeks in the proximal thigh, 3 to 4 weeks in the leg, and 5 to 6 weeks in the distal leg and foot.
2. Lesion in the distal nerve or near the NMJ (i.e., the shortest distance between a lesion and the muscle, as occurs in botulism). Fibrillation potentials develop in just a few days.
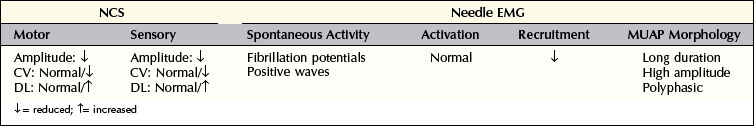
Demyelinating Lesions
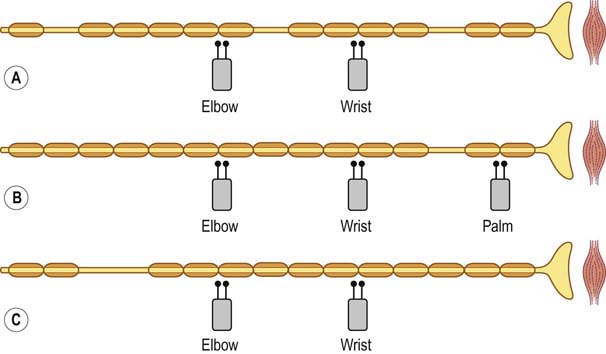
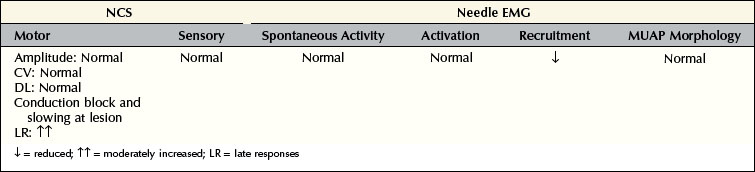
In pure demyelinating lesions (Figure 16–2), the pattern of abnormalities is different from that of axonal loss lesions, and depends on the degree of demyelination. Myelin is essential to maintain the speed of nerve conduction. Accordingly, demyelination first results in marked slowing of CV, as well as prolongation of DLs and late responses. If demyelination is more severe, frank conduction block occurs, with its clinical correlates of sensory loss and weakness associated with blocking of sensory and motor fibers, respectively. Slowing alone, without conduction block, still allows the nerve action potential to reach its destination, albeit more slowly than normal. Pure slowing therefore does not result in any fixed weakness. On the sensory side, pure slowing may result in depressed or absent reflexes and a perception of altered sensation, but not in fixed numbness.
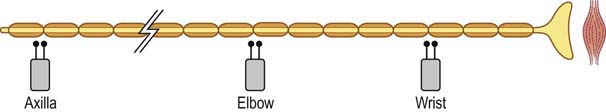
Conduction block nearly always means demyelination; however, in one unusual situation, conduction block may be seen in an axonal loss lesion. If, following a transection, nerve conduction studies are performed above and below the lesion during the first several days, before wallerian degeneration has occurred, a conduction block-like pattern will be seen (Figure 16–3). If the studies are repeated after one week, however, the distal nerve will have degenerated and the apparent block will no longer be present. Some refer to this as a pseudo-conduction block.