Clotting Pathways and Inhibitory Medications
Coagulation Cascade, Anticoagulants, and Thrombolytic Agents
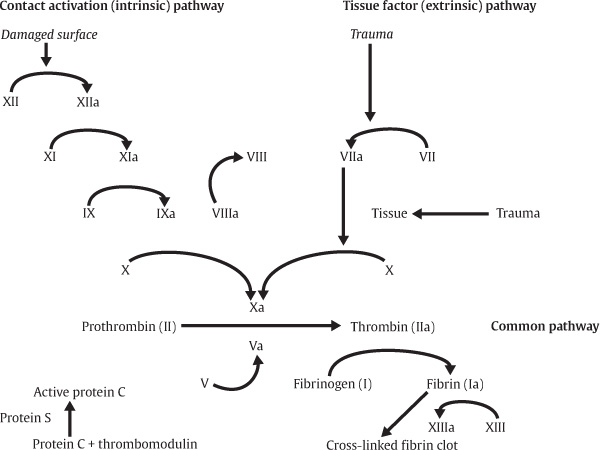
The coagulation cascade is classically divided into intrinsic and extrinsic pathways, which converge to assist the conversion of prothrombin to thrombin IIa, as detailed in Fig. 3.1. Most often, the intrinsic is involved in vessels with damaged surfaces and requires the activation of several factors. Briefly, this contact activation pathway (intrinsic) begins with the formation of a primary complex on collagen by high-molecular-weight kininogen (HMWK), prekallikrein, and factor XII (Hageman factor). Prekallikrein and factor XII are converted to kallikrein and factor XIIa, respectively. The extrinsic pathway, which is directly activated in vessel trauma, has a more significant role in clot formation. This pathway is initiated when injured vascular endothelial cells interact with factor VII, thrombin, plasmin, factors Xa and XII, and tissue factor, which are expressed on stromal fibroblasts and leukocytes (Fig. 3.1).
Heparin
Heparin binds to the enzyme inhibitor factor IIa (antithrombin, AT), causing a conformational change that, in turn, inhibits factor Xa. The formation of a ternary complex between AT, thrombin, and heparin inactivates the thrombin molecule. The inhibition of the AT receptor is size dependent and, for factor Xa inhibition, it is only necessary to have a pentasaccharide binding. The development of low–molecular-weight heparin has allowed more specific binding to factor Xa that has resulted in improved coagulation blockage. One of the main complications associated with heparin is heparin-induced thrombocytopenia (or HIT syndrome). Danaparoid (Orgaran) is a combination of heparan sulfate, dermatan sulfate, and chondroitin sulfate that directly inhibits factor Xa and is often utilized for management of HIT. The absence of any heparin fragments on this drug will reduce the chances of cross-reactivity to under 10%.
Warfarin (Coumadin)
Warfarin (Coumadin) inhibits vitamin K epoxide reductase, an enzyme that recycles oxidized vitamin K to its reduced (active) form; vitamin K participates in the carboxylation of blood coagulation proteins (II, V, VII, X, and proteins C and S). Initially, warfarin promotes clot formation because the half-lives of proteins C and S (anticoagulants) are shorter than those of the calcium-dependent clotting factors (II, VII, IX, and X). Reduced levels of protein S reduce the activity of protein C, which in turn degrades factors Va and VIIIa, which leads to a temporary prothrombotic state. Initiation of warfarin therapy should be bridged with heparin or low-molecular-weight heparin until the INR is therapeutic. Reversal of warfarin therapy includes replacement of clotting factors with fresh frozen plasma (FFP), vitamin K, and, in emergency situations, factor VIIa or VIIIa infusions.
Fig. 3.1 Coagulation cascade.
Thrombolytic Agents (Table 3.1)
Tissue plasminogen activator (tPA), used for stroke lysis therapy, is a serine protease located on the endothelial cell surface that catalyzes the conversion of plasminogen to plasmin. Plasmin, like trypsin, belongs to the family of serine proteases and breaks down fibrin clots. Plasminogen is made in the liver, secreted into the blood stream, and converted into plasmin by tissue plasminogen activator, urokinase plasminogen activator (uPA), and factor XIIa. Plasmin in turn, is deactivated by α2-antiplasmin, a serine protease inhibitor. As with most enzymes, its action depends on its effective binding to a surface receptor.
Table 3.1 Function of Anticoagulation and Antiplatelet Agents
Agent | Function | Dosing |