Extracranial Arterial Occlusive Disease
Etiology
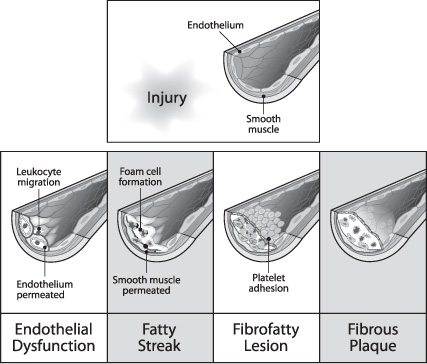
Atherosclerosis, the most common cause of arterial occlusive disease, is a chronic inflammatory response mediated or directed by the endothelial cells, which begins with the deposition of lipids from the blood into the subendothelium (Fig. 17.1). Once the atherosclerotic plaque forms and enlarges, it causes the arterial lumen diameter to decrease in size.
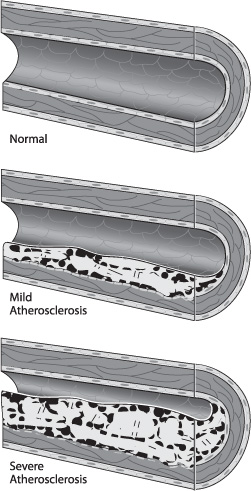
Atherosclerotic plaques can be classified broadly as uncomplicated and complicated. The composition of uncomplicated plaques is relatively uniform, and they are covered by the fibrous cap. Complicated plaques contain areas of hemorrhage, necrosis, ulceration, and calcification (Fig. 17.2). Plaque rupture results from disruption of the endothelium and fibrous cap and leads to embolization through direct release of plaque contents or through the aggregation of platelets to the exposed plaque surface. The posterior wall of the internal carotid artery (ICA) just distal to the common carotid artery (CCA) bifurcation is usually the most susceptible part of the carotid artery to atherosclerosis, and this region is therefore most vulnerable to significant stenosis. Risk factors associated with the advancement of atherosclerosis are diabetes mellitus, dyslipidemia, smoking, and hypertension.1
Fig. 17.1 Genesis of atherosclerotic plaque.
Fig. 17.2 Complicated plaque morphology.
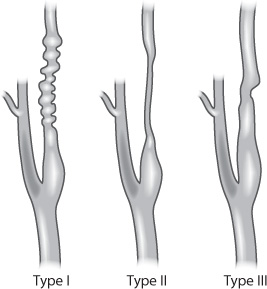
Less common pathological causes of extracranial arterial stenosis include fibromuscular dysplasia (FMD) and certain collagen vascular diseases, such as systemic lupus erythematosus and rheumatoid arthritis. FMD is a nonatherosclerotic, noninflammatory condition that typically affects women aged 15–50 years (Fig. 17.3). It can affect almost every systemic arterial territory, most commonly the renal arteries, followed by the internal carotid arteries.2 The classic angiographic appearance is a “string of beads.” Many times, FMD is discovered incidentally, but it can be symptomatic and lead to arterial dissections and pseudoaneurysm formation.
With increased disease-free survival from head and neck cancers, there is an increasing incidence of radiation-induced carotid stenosis. The specific lesion location depends on where the radiation was targeted. This is in contrast to the normal location of atheromatous plaques, which are usually at arterial bifurcations. A segment of radiation-induced stenosis typically appears long and smooth as compared with its purely atherosclerotic counterpart. The mechanism of stenosis is likely related to direct endothelial injury followed by intimal proliferation, necrosis of the media, periadventitial fibrosis, and acceleration of atherosclerotic changes. An interval of more than five years from irradiation seems to be a predictor for developing significant carotid stenosis.3
Fig. 17.3 Fibromuscular dysplasia in the carotid artery.
Diagnosis
Neurological Examination
Carotid stenosis can cause ischemic neurological symptoms, usually from embolism from the plaque and less commonly from hemodynamic compromise. The common neurological symptoms due to carotid stenosis can range from transient and mild to permanent and fatal. These include transient ischemic attacks (TIA), which are neurological deficits that last for less than 24 hours, reversible ischemic neurologic deficit (RIND), in which symptoms last from one to seven days, and stroke, which implies permanent neurologic dysfunction. The specific symptoms that can arise are dependent on the part of the brain that has decreased blood flow. Another symptom associated with unstable carotid stenosis is transient monocular vision loss. The clinical examination for a patient suspected of having a stroke should be done in a rapid yet thorough manner. If carotid stenosis is suspected as the cause, any of the imaging modalities listed below can be used, depending on their availability and feasibility.
It is imperative, however, that intracranial hemorrhage should be ruled out in the acute period of presentation. Asymptomatic carotid stenosis is often discovered in the presence of a neck bruit, although a bruit is not highly predictive of the presence of a significant stenosis. However, care must be taken when applying a stethoscope or a carotid ultrasound probe on the neck, because some patients may have unstable carotid plaques, which have been reported to embolize during examination.
Diagnostic Imaging Studies and Grading Systems
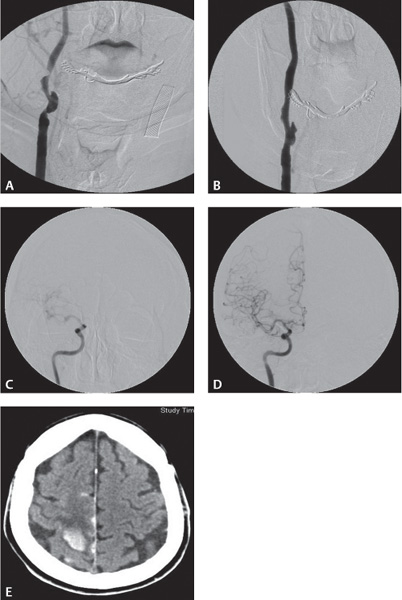
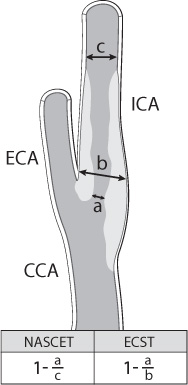
Four common imaging modalities are used to evaluate carotid stenosis: magnetic resonance (MR) and CT angiography, carotid duplex ultrasound, and catheter-based angiography (Fig. 17.4). Each modality has its advantages and disadvantages. Each one, however, is used to determine the percentage of carotid stenosis. The major clinical trials studying the benefit of carotid endarterectomy developed two of the most commonly used techniques to determine the percentage of carotid stenosis. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST) both use the minimal residual luminal diameter as the numerator. As the denominator, the ECST method uses the imagined vessel diameter at the same point if the stenosis were not present. The NASCET method, which tends to yield lower degrees of stenosis compared with the ECST method, uses the diameter of a disease-free distal segment of the artery where the walls of the vessel are parallel (Fig. 17.5).4,5
Fig. 17.4 (A–E) Carotid stenosis using various imaging modalities: CTA, MRA, CUS, Angio.
Fig. 17.5 Determination of carotid stenosis using NASCET/ECST.
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) is used mostly to evaluate the extracranial carotid arteries. It provides good sensitivity (91–99%) and specificity (90–99%) for high-grade stenosis but is less sensitive for the detection of moderate stenosis6 (Fig. 17.4A). However, MRA is costly and time-consuming, and it requires significant patient cooperation. We have found that MRA tends to overestimate the degree of stenosis if the plaque has significant calcifications. There has been recent attention to nephrogenic systemic fibrosis (NSF) with gadolinium-based contrast agents. Although this is a rare but serious skin disorder, it must be taken into account in patients with compromised renal function.
Computed Tomography Angiography
Computed tomography angiography (CTA) is particularly useful for evaluating vessel lumen diameter in the setting of moderate calcification, high carotid bifurcation or extremely tortuous vessels where conventional duplex ultrasonography is not reliable (Fig. 17.4B). The source images obtained in CTA are useful in studying the cross-sectional anatomy of the artery. However, we have found it difficult to evaluate the lumen diameter with CTA in the setting of severe calcification. According to one meta-analysis, CTA had roughly 99% sensitivity in the detection of carotid occlusion.7 The source image reconstructions are helpful in assessing the tortuousity of the artery and the origins if endovascular intervention is anticipated. CTA should therefore be performed with caution in patients with impaired renal function; however, injection protocols have improved so that lower volumes (approximately 80 mL) and non-ionic contrast agents can be used.
Duplex Ultrasound
Ultrasound is a noninvasive, inexpensive test whose accuracy is highly user dependent (Fig. 17.4C). Several parameters, including ICA peak systolic velocity, ICA end diastolic velocity, and carotid ratio (ICA peak systolic velocity divided by the CCA peak systolic velocity), are used to determine the degree of vessel stenosis. Changes in blood flow velocities are typically noted with >50% stenosis. Peak systolic velocity in the ICA ranges from 54 to 88 cm/sec in normal adults. Depending on how the values are adjusted, results can be either very sensitive or very specific. Duplex ultrasonography can be used for higher grades of stenosis, that is, 70–99%, with a sensitivity of 94% and specificity of 89%. False-positive results range from 20% to 41%. Carotid duplex also does not provide information on anatomical configuration, true location, or other areas of distal or tandem stenoses. A set of criteria has been established for ultrasonic classification of carotid stenosis1 (Table 17.1). Care must be taken when applying the carotid ultrasound probe on the neck, as some patients have unstable carotid plaques, which have been reported to embolize during examination.
Angiography
Catheter-based angiography is still considered the gold standard for evaluation of carotid vessels, despite its invasive nature, increased costs, and the potential for procedural morbidity (Fig. 17.4D). As a result, diagnostic angiography is often reserved for cases where noninvasive studies give inconclusive or discordant results or when patients have contraindications to CTA and MRA. For example, patients with both pacemakers and renal insufficiency/failure are often evaluated using diagnostic angiography. In experienced hands, carotid angiography can generally be performed with much less contrast than that needed for CTA. The amount of contrast can be reduced further by injecting only the targeted artery and by using biplane fluoroscopy. If available, rotational three-dimensional angiography is useful for optimal viewing that delineates the arterial stenosis. However, this requires an increased contrast load and is not always necessary.
Table 17.1 Criteria for Carotid Duplex Ultrasound
Grade stenosis | Comment |
Normal ICA | PSV <125 cm/sec; ICA/CCA <2; no visible plaque on grayscale/color imaging |
<50% | PSV <125 cm/sec; ICA/CCA <2; visible plaque or intimal thickening |
50–69% | PSV 125–230 cm/sec; ICA/CCA 2–4; visible plaque |
>70% | PSV >230 cm/sec; ICA/CCA >4; visible plaque |
>99% | Variable velocities due to turbulent flow; may appear occluded on grayscale; color Doppler should show narrow lumen |
Occluded | No patent lumen or flow on grayscale or color Doppler imaging |
Abbreviations: ICA, internal carotid artery; CCA, common carotid artery; PSV, peak systolic velocity.
Evidence-Based Medicine
Several major trials have shown clear benefits for carotid revascularization in carefully chosen patients. Most of these trials compared carotid endarterectomy to medical therapy. More recent trials have attempted to compare carotid angioplasty and stenting (CAS) and carotid endarterectomy (CEA). These trials are summarized in Table 17.2 and Table 17.3. Indications for CAS are evolving, but patients considered high risk for CEA seem to be appropriate for CAS. The high-risk criteria (Table 17.4) seem intuitive, and clinical experience has confirmed these suspicions. We have also learned that high-risk criteria exist for CAS. As experience with CAS and technology is evolving, we see that the high-risk criteria for CAS can be divided into patient-related factors and vascular anatomical factors (Table 17.5).
Preprocedural Preparation
Antiplatelet Therapy
The processes of angioplasty and stenting create intimal injury that promotes thrombosis. Therefore, antiplatelet agents are recommended in the perioperative period. For elective CAS, patients are placed on dual antiplatelet therapy, typically aspirin 325 mg daily and clopidogrel 75 mg daily. Ideally, patients should have started both agents five to seven days prior to the procedure. Patients can also receive 650 mg aspirin and up to 600 mg clopidogrel as a load if more urgent CAS is considered. The onset of action is 20 minutes for aspirin and 4 hours for clopidogrel. Up to 25% of patients are considered “non-responders” to antiplatelet medications, as demonstrated on a variety of platelet function assays. However, there is no general consensus on what level of platelet function must be dysfunctional before thromboembolic risks from stent placement are significantly reduced. Despite this, it is recommended that platelet function be assessed so that patients may be reloaded before their procedure in cases where platelet function is still present.
If CAS is performed on an emergency basis, a glycoprotein IIb/IIIa inhibitor can be administered intravenously once the stent has been deployed and can be followed by a continuous infusion, depending on when the patient can initiate oral antiplatelet agents. (For example, if a patient is loaded orally on aspirin and clopidogrel in the angiography suite during or immediately following CAS, an intravenous bolus of a IIb/IIIa inhibitor may suffice as the onset of action of clopidogrel coincides with the duration of the effect of the IV bolus.)
Anesthesia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree