Endovascular Techniques for Aneurysm Therapy, Arteriovenous Malformation Treatment, and Carotid Artery Stent Placement
Endovascular Aneurysm Therapy
Objectives
The goal of endovascular aneurysm embolization is to occlude the fundus of the aneurysm completely while maintaining the patency of the parent vessel. Partial embolization, both planned and unplanned, is a reasonable option in patients who are unable (or unwilling) to undergo surgery. Partial aneurysm embolization can be performed to protect the dome of wide-necked, ruptured aneurysms in patients who are elderly or critically ill, or who are within the vasospasm period. In some cases, more definitive treatment of a partially coiled, ruptured aneurysm can be performed after the patient recovers.1 In rare cases, partial embolization is performed to make definitive clip reconstruction easier.
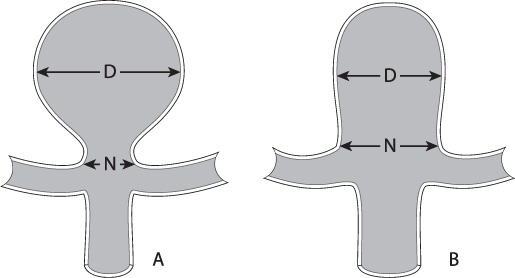
The ability to embolize an aneurysm fully is largely determined by its anatomy, in particular its dome-to-neck ratio (Fig. 8.1). A ratio greater than 2 is generally favorable for coil embolization. If the ratio is less than 2, adjunctive techniques should be considered, such as balloon-assisted coiling, stent-assisted coiling, dual microcatheter coiling, or surgical clipping.2
Medications
Anticoagulation
Stroke is the most common complication encountered in the endovascular treatment of unruptured aneurysms. In acutely ruptured aneurysms, the patient is in a hypercoagulable state as a result of the hemorrhage. In fact, it has been observed that the thromboembolic risk during angiography and interventions is higher in patients with ruptured aneurysms than in patients who undergo elective treatment.3,4 Since patients with ruptured aneurysms often require external ventricular drainage or other supportive surgery, the use of aspirin and clopidogrel is avoided. Heparin is the most common agent used to prevent thromboembolic complications during the coiling of ruptured aneurysms. Other anticoagulants used during aneurysm coiling include bivalirudin, low-molecular weight heparin, and ketorolac (a reversible platelet inhibitor).
Fig. 8.1 The length of the dome (D) divided by the length of the neck (N) is the dome:neck ratio. (A) A favorable dome:neck ratio is 2 or greater. (B) A smaller dome:neck ratio may require adjunctive techniques like balloon or stent assistance for best results.
Heparin
The timing and amount of heparin administered during the coiling of a ruptured aneurysm varies between interventionists. One method is to give a standard bolus of heparin (70 units/kg) at the time the guide catheter is positioned, followed by 1000 units of heparin per hour. The target activated clotting time (ACT) is maintained at around 250 seconds.5 A second method is to administer a half dose (or no dose) at the time the guide catheter is positioned and then to complete the bolus after deploying the first coil.6 A third method is to give no intravenous heparin. Instead, 10,000 units of heparin per liter of saline are flushed intra-arterially through the guide catheter during the procedure. (John Barr, in a 2007 personal communication described this as the Kerber technique.)
Anti-aggregation
For elective treatment of unruptured aneurysms, an 81 mg dose of aspirin (in addition to intravenous heparin) has been advocated to reduce the risk of thromboembolic complications.7 Others, including the authors, advocate the use of clopidogrel prior to the procedure, particularly if the placement of a stent is anticipated.8 In the rare event of a rupture, however, the addition of a non-reversible antiplatelet drug will likely complicate efforts at surgical clot evacuation, clipping, or EVD placement.
Guide Catheter Selection
In younger patients with non-tortuous arch anatomy, the simplest method for obtaining access to the cervical vessels is to use a guide catheter. With standard diagnostic techniques, a 5 or 6 French (Fr) variable stiffness guide catheter can be used to select the cervical internal carotid artery or vertebral artery. Like diagnostic catheters, guide catheters are available in various shapes (Simmons-2, Head-hunter, angled) to accommodate varying anatomy. A 6 Fr guide catheter has a large enough bore to accommodate two microcatheters, a critical feature for balloon-assisted coiling and other dual catheter techniques. A 5 Fr guide catheter will generally accommodate only one microcatheter but can be useful in smaller vessels, where a 6 Fr catheter is occlusive.
With a guide catheter alone, it may be difficult to obtain stable access in the carotid artery in patients with tortuous anatomy. In these patients, a stiffer 80–90 cm guide sheath can be used to straighten out the common carotid artery takeoff from the arch and prevent the system from herniating into the arch during catheterization and coiling of the aneurysm. A guide catheter can be coaxially passed through the guide sheath if more distal access to the internal carotid artery is desired. The combination of a guide sheath, guide catheter, and microcatheter into one system is often referred to as a “tri-axial” system.
Another option is to use an “intermediate” guide catheter. These relatively new guide catheters are softer and more flexible than traditional guide catheters. This allows them to be positioned intracranially while still providing a large enough working channel to accept a single microcatheter.9 Positioning the guide catheter closer to the aneurysm may provide more stability and control of the microcatheter during embolization. Like traditional 5 Fr guide catheters, however, many of these small catheters will not accommodate dual microcatheter techniques.
Microcatheter Selection
In general, coil embolization requires placement of a microcatheter and micro-wire system into the fundus of the aneurysm. A multitude of microcatheters and microwires are available to catheterize aneurysms, each of which has unique properties that make it suitable (or unsuitable) for a particular treatment. It is important to understand the basic features each device so that the most compatible microcatheter/microwire combination is selected for a particular case. For smaller-necked aneurysms, we favor smaller microcatheters to make passing the catheter through the neck of the aneurysm easier. Conversely, for larger aneurysms, microcatheters with a larger inner diameter are preferred to allow for the use of larger coils.
Some microcatheters have a nitinol braid to stiffen them so that they are less likely to herniate out of the aneurysm during coil deployment. Stiffer microcatheters, however, may not track well through tortuous vessels. They may also be more likely to rupture the aneurysm if the tip comes into contact with the wall of the aneurysm.
The tip of the microcatheter should be shaped to mimic the vascular anatomy. Microcatheters pre-shaped at the factory are available in a variety of shapes and angles, but we prefer to custom shape our catheters. In general, a microcatheter will require 30 to 45 seconds in steam to maintain its shape. Shaping of the micro-catheter should be done prior to its introduction into the guide catheter. Some interventionists flush the microcatheter continuously with heparinized saline, while others do not. We advocate having the microcatheter on a continuous heparinized saline flush at all times to help prevent thromboembolic complications and to keep the lubricious inner walls of the microcatheter well hydrated.
Microwire Selection
Microwire selection is similarly complex. Most interventionists use a 0.014″ diameter wire to select the aneurysm. The 0.014″ wire offers a good balance between softness, ability to be torqued, and visibility when compared with larger or smaller diameter wires. The wires most commonly used during neurointerventional procedures are made of nitinol, metal alloy, or platinum. Each of these materials has advantages and disadvantages. In general, the metal alloy and nitinol wires are more responsive to torque than platinum wires, but their tips are stiffer and therefore less delicate. We advocate getting comfortable with how one or two wires behave and using them regularly rather than changing the wire selection for each procedure depending on the anatomy. Some interventionists prefer smaller microwires and will use 0.012″, 0.010″, or even 0.008″ diameter wires. In general, smaller wires are softer and may be less likely to injure a vessel or aneurysm inadvertently. Small wires are especially helpful when attempting to access distal mycotic or perinidal aneurysms. These smaller wires, however, may be less visible on fluoroscopy, so care must be taken when advancing them.
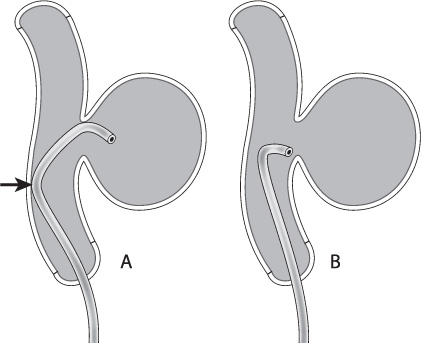
Another important consideration for microwires is the tip shape. Depending on the patient’s anatomy, the tip of the microwire can make the catheterization of the aneurysm either simple or extraordinarily difficult. As with microcatheters, many manufacturers provide pre-shaped tips for purchase. Alternatively, the user can shape the tip of the wire by hand or with a stylette. In general, I use a “headhunter” shape, varying the length and severity of the distal and proximal curves depending on the anatomy to be catheterized (Fig. 8.2). I generally use a nitinol slot-machined microwire that is very responsive to torque (Synchro-2 soft 14 wire, Boston Scientific, Natick, MA). Because of its responsiveness to torque, it maintains a one-to-one relationship to small handheld movements during intervention and hence expedites intracranial navigation. Its tip also maintains its shape well during use.
Fig. 8.2 The double-bend “headhunter” shape (A) will allow the body of the catheter to lean against the back wall of the parent vessel (arrow), providing more support than a simple curve (B).
Microcatheterization Technique
It is extremely important to account for the built-up energy that occurs in a catheter/wire system after each turn in the vasculature. In straight vessels, advancing or withdrawing a device like a wire or catheter at the hub results in an identical movement at the tip. With increasing tortuosity within the vessels, advancing the tip of the device through the curves results in redundancy in the body of the device prior to tip movement. Once the device clears the turn, the stored energy will tend to straighten out the device and spring the tip of the device forward. Therefore, understanding the location of the aneurysm in relation to the vessel anatomy is critical to understanding the safest method of access.
For instance, if the aneurysm is either on or just distal to a turn, there is a high probability that the catheter/wire system will be propelled into the aneurysm during catheterization, with potentially catastrophic results. To avoid these uncontrolled movements, it is safest to advance the catheter and wire past the aneurysm first to release any stored energy, and then pull the system back more proximally to catheterize the aneurysm. In tortuous vessels, we prefer to cross the aneurysm first and then bring the microcatheter back slowly until it faces the aneurysm neck. The microcatheter/wire combination is then re-advanced into the aneurysm. In very small aneurysms I prefer to manipulate the microwire tip within the micro-catheter while redirecting the microcatheter toward the aneurysm. Essentially, the microwire and microcatheter are advanced as one system into the aneurysm.
In general, the catheter should be positioned one-third to one-half the way into the aneurysm fundus to advance coils safely. If the aneurysm is small, it may be safer to position the catheter at its neck rather than in the fundus. In very small aneurysms, coils can even be deployed from the parent vessel across the neck of the aneurysm and into the fundus.
Coil Selection
A multitude of coil choices are available today. The core criteria for coil selection include its diameter, its length, and its 3-D shape. Other considerations include the diameter of the coil wire itself, the coil’s intrinsic stretch resistance, and the specific properties of any “bioactive” coatings that have been applied. Newer framing coils are designed with smaller distal loops that are intended to tumble inside the aneurysm while the larger outer loops form a basket across the neck of the aneurysm. Without these smaller lead loops, the first loop of a large-diameter coil may exit the aneurysm instead of forming a basket inside it.
The 3-D shape of the coil selected should mimic the shape of the aneurysm as closely as possible. Aneurysms with a spherical shape tend to be framed well with “boxlike” coils. Dysmorphic, bilobed, or sausage-shaped aneurysms are better treated with alternative coil shapes.
The initial coils are selected to frame or “paint” the periphery of the aneurysm and cover the neck of the aneurysm. After placing several loops of coil across the neck, the subsequent coils are less likely to herniate through these loops and into the parent vessel. The diameter of the initial framing coil is selected to match the diameter of the aneurysm. Most aneurysms, however, are not spherical. In non-spherical aneurysms, some interventionists will use the mean value of the height, width, and depth of the aneurysm to size the first coil. A framing coil that is significantly over-sized may put excessive tension on the wall of the aneurysm and cause it to rupture. Over-sized coils also tend not to pack well and have a tendency to herniate into the parent vessel. If the diameter of the coil is significantly under-sized, the coil will form its loops but may not apply any tension to the aneurysm wall. In this case, it is possible for the coil to move after detachment and embolize into the parent vessel.
After the framing coil is fully deployed, subtracted angiography should be performed to evaluate its position prior to detachment. It is better to remove a poorly sized framing coil (when possible) and look for a better fit than to struggle with the deployment of subsequent coils. An important improvement in coil design was increasing the length of the framing coils. A coil that is undersized in terms of its 3-D conformation but that is long may still generate adequate friction against the walls of the aneurysm to remain stable after deployment. Long, undersized coils are sometimes helpful in framing dysmorphic, bilobed, or sausage-shaped aneurysms that might otherwise be difficult to coil.
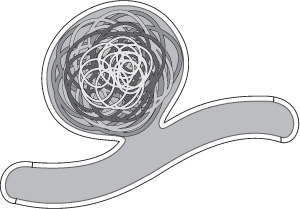
After the framing coil is placed, subsequent coils can either be filling coils or smaller framing coils. If a framing coil is used to fill the aneurysm, it should usually be smaller than the initial framing coil. With the use of additional framing coils, progressively smaller “baskets” are created inside the initial framing coil until the aneurysm is fully embolized. It has been suggested that the result of this technique is a coil mass analogous to Russian Matryoshka dolls10 (Fig. 8.3). Alternatively, softer helical or complex shaped “filling coils” can be used to complete the embolization.
Coils are placed until either the aneurysm no longer fills with contrast during angiography or the microcatheter is pushed outside the aneurysm during coil placement and cannot be replaced after detachment. Most interventionists will perform an angiogram prior to detaching a coil to confirm patency of the parent vessel and to evaluate the efficacy of the coils that have already been deployed. This is often subtracted angiography, but other methods, such as subtracted fluoroscopy, are also acceptable. Another important consideration is to observe coil placement as it relates to the surrounding vessels. One of the earliest signs of coil impingement on a branch vessel includes a delay in emptying, so observation of this finding means that the vessel is compromised and that removal of the deployed coil should be considered. Often the delayed vessel (in emptying) will go on to thrombose on subsequent angiography if the coil is not repositioned.
Fig. 8.3 Framing coils can be used to fill an aneurysm. The first coil (lightest gray) should be the same size as the dome of the aneurysm. Progressively smaller framing coils can then be deployed inside it until the aneurysm is filled. This has been referred to as the “Russian doll” technique.
Detaching Coils
Coils rely on one of three methods for detachment. Most vendors use an electrolytic method for coil detachment. During electrolytic detachment, a charge applied to the end of the coil heats up the material in the detachment zone, causing it to break and detach the coil (Boston Scientific, Micrus, ev3, Terumo).11 Other detachment methods include mechanical (Cook, ev3) and hydraulic systems (Codman, Terumo). The hydraulic system relies on a hypotube attached to an insufflator for deployment. This system seems to have the softest detachment zone of the three systems, making it less likely to push the catheter out of the aneurysm during coil deployment. Care must be taken to avoid kinking or allowing air into the hypotube, as this may prevent the coil from detaching properly.
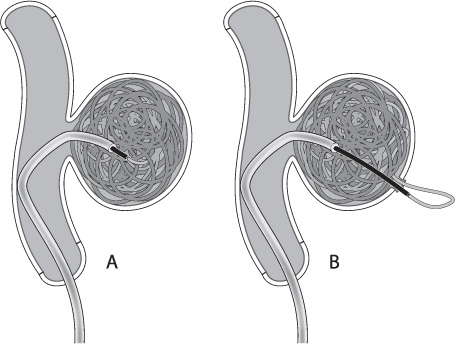
It is important to realize that the detachment zone of all of the coil systems is stiffer than the coil itself. As the relatively stiff pusher is advanced beyond the tip of the microcatheter during coil deployment, the catheter will often back out of the aneurysm. Many times the microcatheter can be carefully re-advanced into the aneurysm over the partially deployed coil. Other times, simply detaching the coil will allow the tip of the microcatheter to fall back into the aneurysm. If the stiff detachment zone is advanced quickly into the aneurysm, it may cause perforation of the aneurysm (Fig. 8.4).12 Be aware of the interaction between the detachment zone and the microcatheter in small aneurysms (or when placing the last coils into a larger aneurysm) to avoid losing microcatheter access. Catheters that fall completely out of a partially coiled aneurysm are often difficult and potentially dangerous to reposition.
Fig. 8.4 (A) Ideally the pusher should extend just past the tip of the catheter during detachment. This is particularly important on the last coil, as deploying the coil inside the microcatheter will often leave a “tail” of coil in the parent vessel when the micro-catheter is removed. (B) If the stiff detachment zone of the coil is deployed too far into the aneurysm, however, it may perforate the aneurysm.
Special Considerations for Very Large and/or Giant Aneurysms
Very large or giant aneurysms remain a significant challenge for cerebrovascular surgery. For endovascular treatment, the most important challenge is the high rate of recurrence.13 To reduce the rate of recurrence, some interventionists (Jacques Dion, personal communication 2004) will advance the microcatheter around the fundus of the aneurysm until it is pointing toward the neck of the aneurysm. Then, if the aspect ratio is favorable, coils are deployed to form a latticework of coil loops across the neck of the aneurysm. This latticework acts like a stent to keep other filling coils from herniating through the neck of the aneurysm and into the fundus.
As a rule, coils will go where the catheter is not. This means that if the micro-catheter is in the most distal part of the aneurysm, the coil loops will push off the wall of the aneurysm and deploy closer to the neck. If the catheter is in the neck, the coils will tend to deploy in the distal fundus of the aneurysm.
With very large or giant aneurysms, adjunctive therapy directed at preserving the parent vessel is important. To reduce recurrence, stent placement within the parent vessel can be performed.14 Alternatively, balloon assistance to increase the density of coil embolization may help reduce recanalization. Lastly, dual micro-catheter techniques can be used for wide-necked aneurysm treatment.15,16
Parent vessel occlusion should be considered when treating very large or giant aneurysms as it reduces the chance of recurrence considerably. An obvious risk to this strategy is ischemia or stroke. Only vessels that have undergone successful balloon test occlusion should be considered for sacrifice. Vessels that do not pass balloon test occlusion should be surgically bypassed prior to sacrifice.
Aneurysmal Mass Effect
Another important consideration is mass effect. The traditional dictum is that a third of patients with symptoms of mass effect (like a third nerve palsy or obstructive hydrocephalus) will worsen after coiling, another third will improve, and the last third will remain unchanged.17 The risk of mass effect progression is increased in the setting of giant aneurysms, so periprocedural management of mass effect symptoms should be considered. Steroids (glucocorticoids) are usually the most effective adjunctive therapy for treating progressive mass effect from aneurysms. In patients who present with mass effect, steroids are generally recommended at the time of treatment and postprocedurally. Surgical clip ligation should be considered in patients with symptomatic mass effect, as clip ligation with evacuation of the aneurysm fundus does appear to reduce mass effect in a greater number of patients than does endovascular treatment.18,19
Adjunctive Endovascular Techniques for Coiling Aneurysms
Dual Microcatheter Technique
In the dual microcatheter technique, the aneurysm is catheterized and coiled as usual using a framing coil, but the coil is not detached. The aneurysm is then catheterized with a second microcatheter, and a second coil is deployed. If both coils are in good position, the coils are detached. If not, one coil or both coils can be recaptured and redeployed.
Balloon-Assisted Aneurysm Embolization
Balloon-assisted coil embolization refers to the positioning and inflation of a balloon in the parent vessel while coils are deployed within the aneurysm.20–22 Once the coil or coils have been deployed, the balloon is deflated, and angiography is performed to verify that the parent vessel is patent. Some interventionists will deflate the balloon between each coil deployment to assess the stability of the coil mass and to shorten the vessel occlusion time. Others will deploy several coils prior to deflation of the balloon. Using the principles of aneurysm clipping with temporary clips, sufficient time should be allowed between balloon inflations to give the brain time to recover (usually 5 minutes between inflations).23 Alternatively, rather than inflating the balloon routinely during coiling, a deflated balloon can be positioned at the aneurysm neck and inflated only as necessary.
Balloon-assisted coiling is especially useful for treating ruptured wide-necked aneurysms, because unlike stent placement, no antiplatelet agents are required. Once a few of the filling coils are in place, the coil mass will usually exert sufficient tension on the aneurysm wall that the balloon can be safely deflated and removed without dislodging the coil mass. A balloon can also slow subarachnoid hemorrhage if the aneurysm ruptures during the procedure.
There are some disadvantages to balloon-assisted coiling. First, balloon-assisted coiling is associated with ischemic complications. When inflated, the balloon blocks normal blood flow through the parent vessel to the brain, resulting in relative hypoperfusion. Stasis both behind and ahead of the balloon can lead to thrombus formation in the parent vessel, particularly if the patient has not received adequate anticoagulation treatment. Balloons can also damage the vessel wall and may cause a dissection.24–27
Technique
In most cases soft, compliant balloons are used for embolization assistance. These balloons are constructed with a wire-valve mechanism. As long as a wire is across the valve, the balloon will inflate and deflate. If the wire is withdrawn, the balloon cannot be inflated, and fluid administered through the hub of the microcatheter will escape through the center lumen. In preparing these balloons, it is important to evacuate any air from the balloon first. To do this, place the deflated balloon in a saline basin and attach a Tuohy-Borst valve connected to a three-way valve with 20 cc and 3 cc syringes. The insufflation solution is a two-thirds or three-fourths contrast-saline mixture. The air is evacuated from the lumen of the balloon by flushing the contrast-saline mixture through the balloon with the wire out. A 0.010″ wire is then passed into the lumen of the balloon, occluding the valve so the balloon can be inflated with a 3 cc syringe. The volume of the balloon is small (approximately 0.2 cc). One will receive tactile feedback through the syringe regarding the amount of pressure needed to inflate the balloon fully (R Chapot, personal communication, 2004). The balloon is semi-permeable, so balloon inflation should be done with the balloon fully submerged in saline (or contrast-saline mixture) to avoid pulling air into the balloon during deflation. A 20 cc syringe can be used to aspirate during deflation to reduce the likelihood of air entering the system. Once the balloon is prepared, it is advanced over the 0.010″ wire to the aneurysm neck, using a high-definition roadmap. The wire is advanced into a distal cerebral vessel to stabilize the balloon and to prevent it from inadvertently sliding into the aneurysm.
An inflated balloon will tend to “sail” forward with the arterial current, so it is often helpful to have an assistant hold the balloon in place while the operator deploys the coils. It will require patience and practice to inflate the balloon correctly and keep the balloon in a stable position across the neck of the aneurysm. Some interventionists will prepare the balloon using only saline to reduce friction between the wire and the balloon (A Molyneaux, personal communication, 2004). Once the balloon is in position, it can be inflated with a contrast/saline mixture. To remove the saline from the balloon, remove the wire and flush it with a contrast-saline mixture. The wire can then be reintroduced to inflate the balloon.
Though these balloons are soft, it is important to note that their reference diameter is usually 4 mm. In vessels less than 2 mm, try to position part of the balloon in a larger branch to allow the balloon to herniate into that branch if inadvertent over-inflation occurs. In a prospective trial examining prophylactic balloon inflation to prevent vasospasm, all of the vessel ruptures occurred in small vessels (less than 2 mm).28–30
Use of Liquid Embolics for Treatment of Aneurysms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree