Complications of Cancer Therapy
Jasmin Jo
David Schiff
INTRODUCTION
Neurologic complications of cancer therapy are relatively common and may be disabling. Understanding these complications may mitigate or halt progression of injury to the nervous system through early recognition and prompt management. In this chapter, we will discuss neurotoxicities associated with the various agents used in the treatment of cancer.
NEUROLOGIC COMPLICATIONS OF RADIOTHERAPY
Cranial and spinal radiation used to treat primary or secondary central nervous system (CNS) tumors as well as for prophylaxis in certain systemic malignancies is a recognized cause of neurologic complications. The cranial and peripheral nerves are also vulnerable to adverse effects when included in the radiation field for CNS or non-CNS tumors. The risk for radiation injury increases with higher total dose and fractions, larger treatment volume, and coadministration of chemotherapy. Patients younger than 10 and older than 70 years old are more susceptible to complications of radiotherapy (RT).
PATHOBIOLOGY
Tissue injury from RT is categorized in sequential stages: acute (<1 month), early-delayed (1 to 6 months), and late-delayed reactions (>6 months). The timing is helpful in predicting reversibility of tissue damage. Capillary injury and leakiness leading to edema is the mechanism during acute injury. Late injury is generally associated with permanent tissue damage. In the brain and spinal cord, the proposed mechanism for late effects is a combination of vascular injury involving the small- and medium-sized vessels, demyelination with loss of oligodendrocytes, and immunologic response to antigens released from damaged glial cells. The pathologic end state is radiation necrosis, involving coagulation necrosis and gross demyelination. Tissue atrophy is seen upon long-term follow-up. Table 105.1 summarizes RT injuries and their corresponding manifestations based on location.
TABLE 105.1 Neurologic Complications of Radiotherapy | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
CLINICAL MANIFESTATIONS AND DIAGNOSIS
Brain
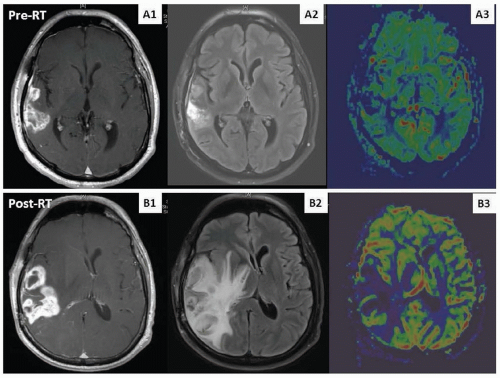
Acute encephalopathy, consisting of somnolence, headache, nausea, vomiting, and exacerbation of preexisting deficits, usually develops days after initiation of RT and generally responds to corticosteroids. No specific neuroimaging findings are associated with acute injury. Early-delayed complications include the somnolence syndrome, characterized by drowsiness, fatigue, anorexia, irritability, and transitory cognitive disturbances primarily affecting attention and short-term memory. Neuroimaging at this time may be unchanged or show increased edema and contrast enhancement within the tumor bed that mimics tumor progression, termed pseudoprogression (Fig. 105.1). The exact frequency with which this phenomenon occurs is difficult to estimate because of varying criteria defined in different reports. However, it occurs in at least 10% to 20% of patients with glioblastoma receiving concurrent RT and temozolomide; it manifests most commonly 1 to 3 months posttreatment (although may occur later) and usually improves within a few months. Late injury is associated with focal radionecrosis (causing seizures, focal neurologic symptoms, and signs of increased intracranial pressure) and leukoencephalopathy (leading to cognitive dysfunction and severe dementia). Diffuse white matter changes and atrophy are seen in the neuroimaging in nearly all cases. Magnetic resonance imaging (MRI) does not distinguish between radiation necrosis and tumor progression. Metabolic and
perfusion imaging studies may help discriminate the two entities, but surgery remains the gold standard to confirm the diagnosis.
perfusion imaging studies may help discriminate the two entities, but surgery remains the gold standard to confirm the diagnosis.
Spinal Cord
Radiation-induced damage to the spinal cord is categorized into early-delayed (6 weeks to 6 months) and late-delayed (>6 months) myelopathy. Acute worsening should prompt investigation for intratumoral hemorrhage or tumor progression. Early-delayed RT myelopathy is characterized clinically by Lhermitte sign that typically resolves spontaneously, with no distinctive imaging finding. Patients with late-delayed radiation myelopathy often presents with Brown-Séquard syndrome, spastic paraplegia with impaired sensory and autonomic functions. The condition may begin abruptly or insidiously and is most often irreversible. MRI demonstrates increase T2 hyperintensity and enhancement in the affected spinal cord levels.
Peripheral Nervous System
Any cranial nerves may be involved if included in the radiation field. The hypoglossal nerve is the most vulnerable followed by the vagus nerve and the recurrent laryngeal nerve, believed to be due to absorption of higher amount of energy from RT to the neck region. Optic neuropathy results in painless and progressive visual loss or visual field constriction. The oculomotor, trochlear, trigeminal, abducens, and facial nerves are less vulnerable and can be affected from focal RT for skull base tumors. Trigeminal neuropathy is quite rare and may result from radiosurgery for vestibular schwannoma and trigeminal neuralgia. Radiation-induced injury to vestibulocochlear nerve is rare, except in RT for acoustic neuroma. Hearing impairment, especially at high frequency, may be due to changes in the cochlea and/or retrocochlear auditory pathway. Permanent damage to cranial nerves is rare and usually occurs due to delayed effect.
RT-induced brachial and lumbosacral plexopathies may result from treatment of breast, lung, and pelvic cancers. The usual presentation includes paresthesia and hypesthesia, followed by weakness and amyotrophy. Pain is usually relatively mild and occurs late in the course, as opposed to severe pain in patients with malignant plexopathy. Myokymia seen on electromyography and hypointensity in T1- and T2-weighted sequences and absence of mass on MRI suggest RT plexopathy.
Indirect Complications to Nervous System
RT can indirectly cause endocrine dysfunction such as hypothalamic-pituitary impairment resulting in hypothyroidism, hypogonadism, hyperprolactinemia, and panhypopituitarism; vascular damage leading
to stroke, hemorrhage, vascular malformations, and rarely, SMART syndrome (stroke-like migraine attacks after radiation therapy); and secondary tumors such as meningioma, glioma, and sarcoma.
to stroke, hemorrhage, vascular malformations, and rarely, SMART syndrome (stroke-like migraine attacks after radiation therapy); and secondary tumors such as meningioma, glioma, and sarcoma.
TREATMENT AND PREVENTION
Corticosteroid (dexamethasone, intravenous [IV] or oral, initially at 16 mg/day) generally reverses symptoms from acute and earlydelayed injuries but has variable benefit in radiation necrosis. Antivascular endothelial growth factor (VEGF) agents such as bevacizumab (10 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks IV) have demonstrated beneficial effects in patients with cerebral radiation necrosis. In rare occasions, surgical resection of cerebral radiation necrosis is indicated, providing both therapeutic and diagnostic benefits. The use of memantine at 20 mg/day within 3 days of initiating RT for 24 weeks results in delayed time to cognitive decline and reduced rate of decline in memory, executive function, and processing speed in patients with brain metastases receiving whole brain RT [Level 1].1 Hippocampal sparing during brain RT may also reduce the incidence of neurocognitive injury. Pain management and physical therapy are important aspects in the management of RT-induced plexopathy. Hyperbaric oxygen and anticoagulation may ameliorate the effects of radiation necrosis, but their efficacy is limited.
OUTCOME
Recovery of symptoms is typically expected in acute and earlydelayed RT injuries. However, delayed complications of RT are progressive and irreversible, leading to poor outcome and quality of life for long-term survivors.
NEUROLOGIC COMPLICATIONS OF CHEMOTHERAPY
Cytotoxic chemotherapeutic agents may cause toxic effects to the peripheral and central nervous system, often leading to reduction or cessation of treatment. The severity depends on the treatment dose, duration, route, existing comorbidities, and coadministration of other neurotoxic agents.
PATHOBIOLOGY
The mechanism of chemotherapy-induced peripheral neuropathy (CIPN) depends on the cytotoxic agents used. Antimitotic agents, such as vinca alkaloids and taxanes, disrupt microtubule-based axonal transport leading to length-dependent axonal injury. Platinum agents such as cisplatin cause neuropathy by apoptosis of sensory neurons in dorsal root ganglion, whereas oxaliplatin additionally causes transient Na-gated channel dysfunction resulting in altered nerve excitability, particularly refractoriness. Despite the presence of the blood-brain barrier (BBB), the CNS remains susceptible to neurotoxicity effects if the protective barrier is breached by direct effects of tumor through endothelium damage or by RT, if the agent crosses the intact BBB, or if the agents are administered directly to the cerebrospinal fluid (CSF) or into the cerebral vasculature. Chemotherapy can also cause damage to neural progenitor cells responsible for neurogenesis and maintenance of white matter integrity.
CLINICAL MANIFESTATIONS
Central Nervous System
Chemotherapy agents such as ifosfamide, high-dose methotrexate, and procarbazine can cause acute toxicities occurring during or few days after treatment, characterized by confusion, hallucination, seizures, and drowsiness. A cerebellar syndrome may develop from high-dose cytarabine. Intrathecal (IT) methotrexate and cytarabine can cause aseptic meningitis and myelopathy. IT vincristine causes severe neurotoxicity that is nearly always fatal and must be avoided. Posterior reversible encephalopathy syndrome (PRES), manifesting as acute or subacute onset of headache, seizures, confusion, and visual changes, has been reported from cisplatin, cyclophosphamide, high-dose corticosteroids, and gemcitabine. Chronic leukoencephalopathy, characterized by progressive personality change, dementia, ataxia, and incontinence, may occur months to years following methotrexate, especially when given during or soon after whole brain RT. Therefore, whole brain RT should be administered after systemic or IT methotrexate.
Peripheral Nervous System
CIPN typically presents with symmetric distal paresthesia, loss of proprioception and vibratory sense, and loss of ankle reflexes. Distal motor weakness and autonomic dysfunction including atonic bladder, impotence, and orthostatic hypotension may also occur with certain agents (Table 105.2). Vestibulocochlear toxicity, with hearing loss, vertigo, and ataxia, is associated with cisplatin. Acute cold-induced dysesthesia involving the distal extremities, throat, mouth, or face occur commonly with oxaliplatin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree