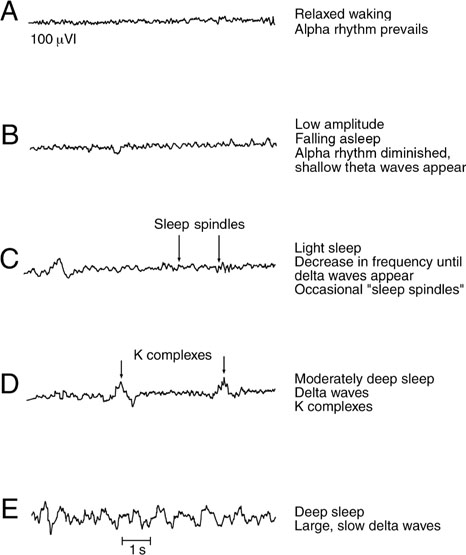
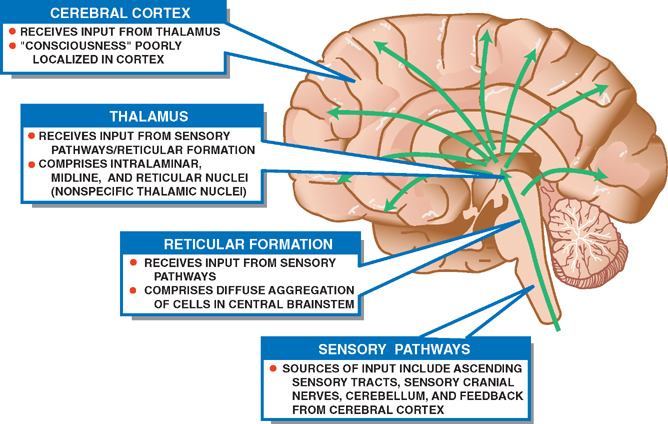
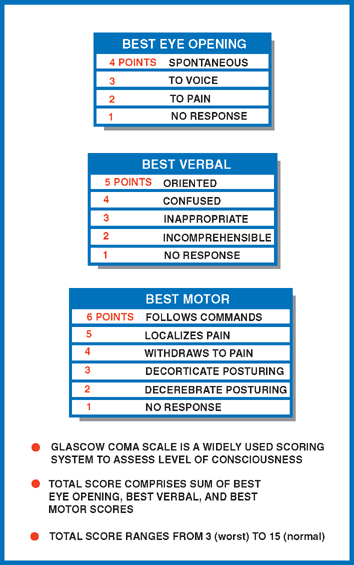
21 Consciousness Consciousness means awareness of self and environment. The modern era of the study of consciousness began in the 1920s when it was discovered that awake and sleeping states were associated with distinctive electroen-cephalographic (EEG) patterns. These early EEG records showed that alertness was associated with low-voltage fast activity; sleep, with high-voltage slow activity (Fig. 21.1). Later experiments demonstrated that the EEG pattern of sleep was replaced by a pattern of wakefulness after stimulation of the brainstem reticular formation. Further, this change resembled the EEG patterns during the transition between drowsy and awake states. Although these studies suggested that the reticular formation exerted broad and diffuse influence over the cerebral cortex, the anatomic and physiological mechanisms remained unclear. In particular, the connecting pathways between the reticular formation and the cerebral cortex were not well understood. Two observations proved pivotal. First, it was noted that stimulation of the reticular formation activated the EEG, even after the placement of destructive lesions in the ascending sensory pathways (e.g., the medial lem-niscus and the spinothalamic tract). Second, ascending sensory pathways conducted impulses normally, even when destructive lesions were placed in the reticular formation. These observations implied that a second ascending system, anatomically and physiologically distinct from the “specific sensory pathways,” carried impulses generated in the reticular formation. Two separate ascending pathways evidently existed: (1) a long ascending pathway concerned with specific sensory modalities and (2) an ascending reticular activating system (ARAS) concerned with arousal. Further studies showed that both systems synapse in the diencephalon before reaching the cerebral cortex. However, the long ascending pathway synapses in “spe-cific thalamic nuclei” (ventral posteromedial and ventral posterolateral nuclei), whereas the ARAS synapses in “nonspecific thalamic nuclei” (intralaminar, midline, and reticular nuclei). Furthermore, although both systems terminate in the cerebral cortex, the long ascending pathway terminates in the primary sensory cortex; the ARAS terminates diffusely throughout the cerebral cortex. Clearly, then, the reticular formation, which was discussed in Chapter 6 in the context of the anatomy of the brainstem, is a morphological designation. It refers to a diffuse network of neurons with a wide variety of functions and connections. By contrast, the ARAS is a functional designation: it refers to a system of neurons concerned with arousal, some of which are found in the reticular formation. Fig. 21.1 Characteristic electroencephalographic (EEG) patterns associated with awake and sleeping states. See Fig. 21.2. Several levels of the neuraxis contribute to the ARAS: (1) afferent sensory pathways, (2) the reticular formation, (3) the thalamus, and (4) the cerebral cortex. Widespread sources of sensory input are projected onto the reticular formation. These sensory stimuli possess varying capacities to arouse—some weak, such as a gentle whisper; some strong, such as a bucket of cold water thrown on a sleeping subject’s head. Important sources of sensory input include (1) ascending tracts of the sensory system, (2) sensory cranial nerves, (3) the cerebellum, and (4) the cerebral cortex. Cortical input provides an important feedback mechanism that modifies other sources of sensory input. The reticular formation is a diffuse aggregation of cells in the central brainstem that possesses an unusually wide range of neural connections. These connections involve the reticular formation in several functions, including motor control, control of the respiratory and cardiovascular system, sensory control, and consciousness. Reticular neurons that contribute to the ARAS are concerned with consciousness. They receive sensory inputs from multiple regions that project to the thalamus through long axons. The thalamus contains three sets of functionally related nuclei: (1) the “specific thalamic nuclei,” which receive sensory and motor input and project to specific areas of the cerebral cortex; (2) the “association thalamic nuclei,” which receive input from other thalamic nuclei and project to association areas of the cerebral cortex; and (3) the “nonspecific thalamic nuclei,” which receive input from the ARAS and project to large areas of the cerebral cortex. The nonspecific thalamic nuclei, which contribute to the ARAS, are the intralaminar, midline, and reticular nuclei. As noted, cortical electrical activity as recorded by the EEG correlates well with the behavioral expression of consciousness—the awareness of self and environment. The awake person demonstrates low-voltage, high-frequency EEG activity (α and β; > 8H3), whereas the sleeping patient demonstrates high-voltage, low-frequency EEG activity (8 1 to 4H3). Activation of the cerebral cortex is dependent on thalamocortical innervation. Specific sensory, motor, and cognitive functions have long been known to “localize” to specific areas of the brain. By contrast, no specific region of the brain is responsible for initiating or maintaining arousal. Rather, all areas of the cortex appear to contribute equally to consciousness. Thus, consciousness requires an intact cerebral cortex, and cerebral lesions, if sufficiently large, may impair consciousness. The degree of impairment is proportional to the size of the cerebral lesion and bears little relation to what area of the cortex has been lesioned. Fig. 21.2 Ascending reticular activating system. Consciousness is the awareness of self and environment. Unconsciousness, it follows, is its opposite: a lack of awareness of self and environment. States of consciousness and unconsciousness occur along a continuum of varying levels of awareness, where wakefulness and coma represent two extremes. Behaviorally, coma is the complete lack of awareness of self and environment. Pathologically, it may be due to one of two possible conditions: (1) a bilateral cerebral hemisphere disturbance or (2) a dysfunction of the ARAS. A logical diagnostic approach in the comatose patient begins with a history of the present illness from friends, relatives, police, or paramedics and a specially tailored neurological examination. Primarily in the basis of the clinical signs elicited by exam, an anatomic localization may then be deduced. Finally, on the basis of primarily historical clues, the clinician must ask what specific pathological process is most likely responsible and what therapeutic intervention is most appropriate. The purpose of the neurologic examination in the stu-porous or comatose patient is to define the level of consciousness and to localize the site of the offending lesion. Levels of consciousness are often described, in order of decreasing intensity, in terms of wakefulness, obtun-dation, stupor, and coma. Their determination and differentiation are largely subjective. A more objective and reproducible approach is to observe the patient’s response to a series of increasingly intense stimuli. The Glasgow Coma Scale is a commonly used method for testing and recording the consciousness level (Fig. 21.3). Localizing the site of the offending lesion in the comatose patient may be reduced to the interpretation of four clinical signs: respiratory pattern, pupillary response, eye movements, and motor response.
Ascending Reticular Activating System
Sensory Pathways
Reticular Formation
Thalamus
Cerebral Cortex
Approach to the Comatose Patient
Neurologic Examination
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








