Chapter 125 Neurovascular Decompression in Cranial Nerves V, VII, IX, and X
The compression of specific cranial nerves at their entrance to or exit from the brain stem has been linked to a number of clinical conditions. Tic douloureux or trigeminal neuralgia is the most common, and perhaps the best described and investigated, of these cranial nerve disorders. Dandy was the first to observe, in 1932, that vascular compression of the trigeminal nerve in the posterior fossa might be the cause of tic douloureux.1 Gardner and Sava later described vascular compression of the seventh cranial nerve in more than half of the patients they operated on via the posterior fossa for hemifacial spasm, the motor equivalent of trigeminal neuralgia.2 However, it was not until Jannetta applied the operating microscope to the systematic study of these problems that the truly remarkable incidence of pathologic vascular compression of cranial nerves at their entry to or exit from the brain stem was appreciated.3 Based on his findings, Jannetta devised an operative technique (microvascular decompression) to displace these vessels from the affected nerve without sacrificing neural integrity.3,4 This technique has been used to relieve the specific cranial nerve syndromes successfully in the majority of patients treated. This chapter discusses the individual cranial nerve vascular compressive syndromes and the decompression techniques that can be used in treating them.
Fifth Cranial Nerve
Clinical Presentation
Trigeminal neuralgia is characterized by a stereotypical clinical syndrome. Patients suffering from trigeminal neuralgia exhibit brief, intense paroxysms of pain confined to one or more divisions of the trigeminal nerve. These attacks are usually triggered by light cutaneous stimuli within the trigeminal territory. The pain typically is described as an intense stabbing or electrical shock sensation that patients frequently describe by rapidly flinging open their hands to indicate the paroxysmal nature of the jolts of pain. The pain is often more pronounced during the day, and many patients are pain free or have markedly fewer episodes at night. The most common areas of involvement are in the second and third trigeminal divisions, specifically anteriorly and in the region of the mouth. Because the mouth is a common site of symptoms, patients may undergo unnecessary dental surgery without achieving relief before the diagnosis of trigeminal neuralgia is made. Patients classically guard their faces, refuse to be touched, and avoid shaving, washing, applying makeup, chewing, and brushing their teeth, because any of these actions may provoke an attack. Over time, patients frequently report an increase in the number of attacks, a reduction of pain-free periods, and occasionally sensory disturbances in the involved trigeminal distribution or distributions.4,5
Differential Diagnosis
Trigeminal neuralgia is occasionally the presenting complaint of a patient with multiple sclerosis. Trigeminal neuralgia occurs in 1% to 3% of patients afflicted with multiple sclerosis. Similarly, 2% to 4% patients with trigeminal neuralgia eventually are found to have multiple sclerosis.6 The clinical picture and site of the pathologic process (the root entry zone of the nerve) appear to be identical in idiopathic trigeminal neuralgia and multiple sclerosis patients. However, in multiple sclerosis, the cause of neuralgia is intrinsic demyelination of the nerve, which is not due to extrinsic vascular compression. Subsequently, microvascular decompression does not benefit multiple sclerosis patients, and a neural destructive procedure should be used to treat medically refractory cases.
Various other conditions must be considered in the differential diagnosis of trigeminal neuralgia. These include herpes zoster (postherpetic neuralgia), dental disease, orbital disease, temporomandibular dysfunction, and temporal arteritis, as well as post-traumatic neuralgias. None of these typically presents with the classic lancinating paroxysmal pain of trigeminal neuralgia. Once the clinical diagnosis of trigeminal neuralgia is made, magnetic resonance imaging (MRI) should be performed to rule out demyelinating disease, infection, inflammatory processes, vascular malformations, and neoplasms.
Medical Therapy
After the diagnosis of trigeminal neuralgia is made, a trial of 100 mg of carbamazepine given twice a day is initiated. The dose is increased by 100 mg every other day until pain control is achieved or toxicity develops. Patients are instructed to take this medication on a full stomach and to have monthly hematologic studies performed so that toxicity can be detected early. In this manner, good control of the pain can be achieved in the vast majority of patients.7,8 Patients who are allergic or intolerant to carbamazepine can be treated with phenytoin, oxcarbazepine, clonazepam, and baclofen. Although gabapentin is frequently promoted for this indication, no studies have been published showing its efficacy, and it has rarely proved useful in our clinical experience. Surgery is reserved for patients whose pain is refractory to medical treatment or who develop medication-related side effects.
Indications
If the patient meets the typical clinical picture and fails medical therapy, surgical treatment options include microvascular decompression, selective percutaneous lesioning (with radiofrequency thermal coagulation or glycerol chemoneurolysis), or stereotactic radiosurgery of the trigeminal nerve. It is our practice to explain the relative benefits and risks of the surgical treatment options and assist the patient in choosing among these procedures (Table 125-1). We believe that microvascular decompression is the procedure of choice for the treatment of trigeminal neuralgia in otherwise healthy and relatively young (generally younger than 70 years) patients.9
Table 125-1 Relative Benefits of Percutaneous Trigeminal Neurolysis, Radiosurgical Trigeminal Neurolysis, and Microvascular Decompression of the Trigeminal Nerve for the Treatment of Trigeminal Neuralgia
| Procedure | Benefits | Drawbacks |
|---|---|---|
| Percutaneous trigeminal neurolysis | ||
| Stereotactic radiosurgery | ||
| Microvascular decompression |
Operative Positioning
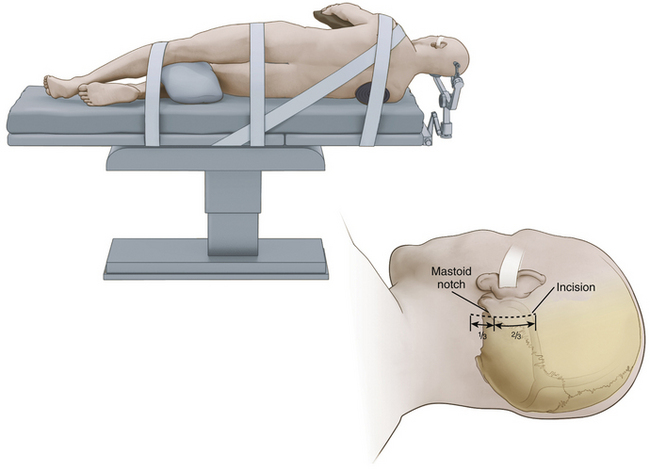
While we frequently use the sitting position for microvascular decompression procedures (as described in detail later), others often use a lateral or park bench position (Fig. 125-1).10 Briefly, for lateral or park bench positioning, the patient is induced, intubated, and placed under general anesthesia, and the head is secured in three-point pin fixation. The patient is then placed in the lateral decubitus position with the unaffected side down. A gel roll is placed in the axilla of the dependent side, all pressure points are padded, and the nondependent arm is supported by a padded armrest. The neck is then secured in a slightly flexed position, and the head is turned 10 to 20 degrees from the affected side to provide optimal exposure and a clear line of sight to the pathology during surgery. The vertex of the head is then positioned based on the site of vascular compression.10 For decompression of the fifth cranial nerve, the vertex of the head is maintained parallel to the floor. For decompression of the lower cranial nerves, the vertex of the head is turned 10 to 20 degrees toward the floor. The patient is secured to the operative table with 3-inch tape and/or Velcro straps, and the shoulder of the affected side can be gently taped caudally to provide additional working space. The remaining portions of the operation are similar to those described later, including incision, dural opening, intradural dissection/decompression, and closure.
Anesthetic Considerations
To detect air embolization, a Doppler precordial detector and an end-tidal carbon dioxide monitor are used because they can detect even minute amounts of air. This detection allows the anesthesiologist to raise the venous pressure and prevent further entrainment of air, avoiding massive air embolization. The effectiveness of these methods of intravenous air detection is such that we abandoned the use of central venous pressure catheters for this type of surgery. The central venous pressure catheter was originally inserted to allow the aspiration of air trapped in the right atrium. Small amounts of air, however, do not sequester in the atrium but pass through the heart into the pulmonary circulation. If detected at its earliest stages, raising the venous pressure to prevent further entrainment is sufficient.
Positioning Preferences
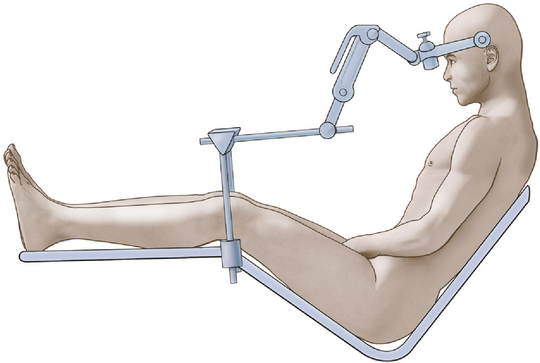
We achieve the sitting position using a pin-fixation head holder for rigid fixation of the skull. The patient’s head is rotated 15 to 30 degrees to the ipsilateral side. The head is flexed gently to provide ample room for placing one or two fingers beneath the patient’s chin (Fig. 125-2). The elevation (or angle) of the back of the table is such that the patient is actually in a semisitting or slouched position, although higher elevation of the backrest may be necessary in the older patient with a less flexible neck.
Operative Procedure
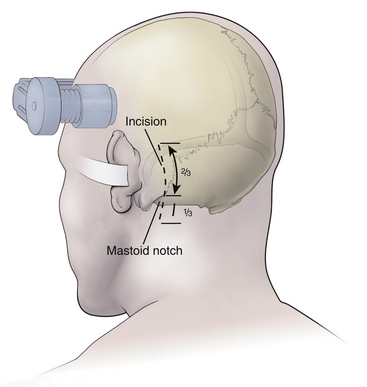
After the patient is satisfactorily anesthetized and positioned, the operative table is angled an additional 15 to 20 degrees to the ipsilateral side so that the surgeon approaches the patient at an angle of approximately 45 degrees from the midline. The hair is shaved only from the posterior quadrant of the head on the affected side, and sterile preparation and draping are performed. The incision is vertical and located 3 to 5 mm medial to the mastoid notch (Fig. 125-3). We use a linear incision approximately 8 cm long that is centered two thirds above and one third below the mastoid notch.
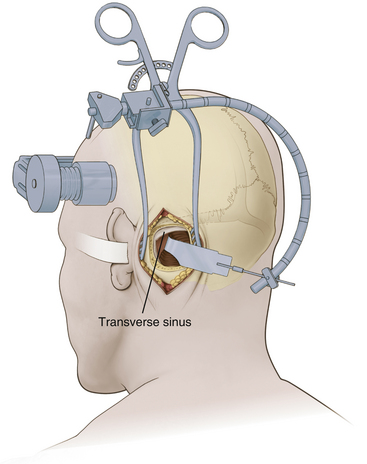
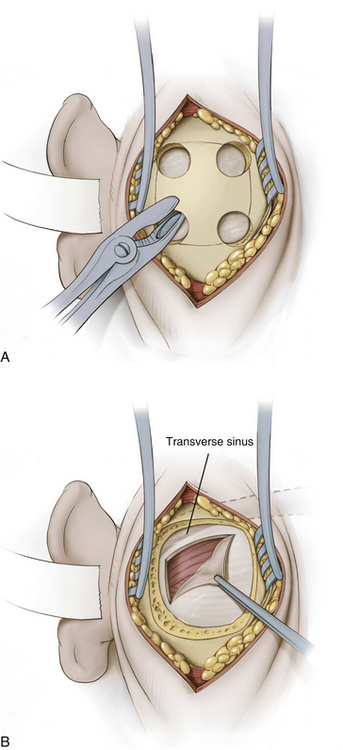
A modified Weitlaner retractor (Apfelbaum retractor) that serves as the base for a self-retaining brain retractor (Codman & Shurtleff, Raynham, MA) is then placed within the wound (Fig. 125-4). The retractor is secured by placing a gauze sponge through the loops of the handle and clipping it to the drape above the patient’s head to provide good three-point fixation and achieve adequate stability for the retractor arm. Several bur holes are made and enlarged into a circular craniectomy (2.5-3 cm) (Fig. 125-5). The craniectomy should extend superiorly to the transverse sinus and laterally to expose the sigmoid sinus. The lateral extension of the bony opening often carries the craniectomy over mastoid air cells, which are thoroughly waxed at the completion of the craniectomy. Care is taken to displace the dura as the rongeuring proceeds to avoid entering the dura or venous sinuses. Bridging veins may also be encountered and have to be coagulated.
The dura then is opened in an inverted L-shaped manner 3 to 5 mm parallel to the sigmoid and transverse sinuses (Fig. 125-5). The dura can be further opened in a T shape at the superior corner if increased exposure is needed. The dura is then secured with tenting sutures superiorly and laterally to retract the sinuses slightly and complete the exposure.
Occasionally, adhesions or bridging vessels are encountered along the superior posterior margin of the cerebellum along the transverse sinus. These must be divided sharply to free the cerebellum. A flexible retractor arm is then placed on the retractor base. This retractor arm should be positioned so that a gentle arch is formed and sharp kinks and bends are avoided (Fig. 125-4). Its tension is adjusted so that it remains in any position in which it is placed but can be readily moved without undue force. A specially shaped retractor blade that has an elongated finger at its superior margin (Codman & Shurtleff) is used (Fig. 125-4). The purpose of the finger is to allow deeper retraction in the vicinity of the trigeminal nerve while avoiding deep retraction and potential injury to the seventh and eighth cranial nerves. A narrow retractor blade could achieve the same depth of exposure superiorly but might penetrate into the cerebellum. Once the retractor blade is in place, the superior lateral margin of the cerebellum is retracted in a medial-to-inferomedial direction, and the operating microscope is brought into use.
With the microscope in place, the cerebellum is gently retracted further medially. The approach should be angled as sharply as necessary to visualize the cerebellar surface. Adhesions and small bridging vessels are lysed as the cerebellum is retracted. Once the lateral extent of the cerebellum is visualized, the angle of approach is changed to follow the petrous bone anteriorly. McLaughlin et al.10 refer to this change as “turning the corner” and emphasize the necessity of doing this under direct vision. At this point, the petrosal vein can be identified. This vein frequently is encountered two thirds of the way from the dura to the trigeminal nerve, but great variability exists, and it may be absent or positioned close to the nerve. It often consists of two channels that form a Y-shaped bifurcation just before entering the dura. To permit adequate retraction and deeper dissection, the petrosal vein may need to be coagulated and divided sharply.
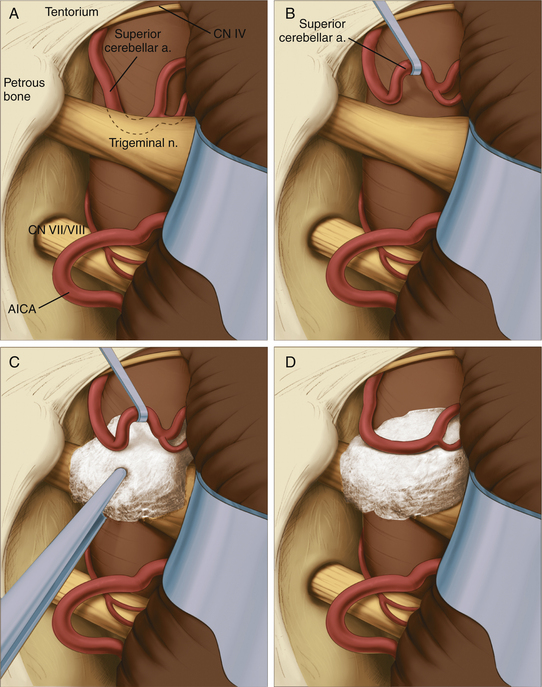
The site of pathology is typically at the brain stem, and vessels impinging distally on the nerve are not usually the cause of the problem. The typical situation involves the superior cerebellar artery looping down in front of the nerve and emerging from the nerve dorsally at the point where the nerve exits the brain stem (Fig. 125-6). Opening the arachnoid widely allows full inspection of the entire circumference of the nerve at the brain stem. The first vessel seen may not be the only vascular channel involved in the neurovascular compression, because in a significant number of cases, multiple vessels have been encountered. It is also necessary to open the arachnoid anterior to the nerve to allow proper placement of the prosthesis.
Jannetta microsurgical instruments (V. Mueller & Co., Chicago, IL), Rhoton dissectors (V. Mueller & Co.), or Apfelbaum dissectors (Integra Life Sciences, Plainsboro, NJ) are of sufficient length and properly fashioned to allow adequate vision throughout the operation. Various microsurgical scissors, including the Kurze left and right pistol-grip scissors (V. Mueller & Co.) and straight and angled bayoneted microscissors, are also employed. Once the arachnoid has been opened fully and the area has been inspected completely, the exact nature of the compression can be determined. A microdental mirror (warmed in hot saline to reduce fogging) may be useful in inspecting the region anterior to the nerve. This trigeminal nerve exposure is carried out directly over the seventh and eighth nerves (Fig. 125-6). The presence of these nerves must be kept in mind to avoid traumatizing them during the dissection or while inserting or removing instruments. The arterial loops that are found are then carefully dissected free of the trigeminal nerve.
When the superior cerebellar artery is the problem, the intent is to elevate it to a horizontal rather than vertical orientation and to displace it upward and away from the nerve (Fig. 125-6). This elongated vessel may have small branches going to the brain stem. Normally, these branches do not present a problem in the elevation of the vessel, as long as their position is kept in mind. Venous channels above or below the nerve are dissected away from the nerve and are coagulated and divided. The coagulation of these vessels is facilitated by the use of small up-and-down–angled bipolar forceps that prevent the spread of current to the adjacent neural structures. In the case of vessels compressing the nerve inferiorly, they must be displaced further inferiorly away from the nerve. In all cases, it is important to avoid kinking the arterial channels as they are repositioned.
To secure these vessels free of the nerve, a small prosthesis is inserted between the artery and the nerve (Fig. 125-6). For this purpose, we use either Ivalon or shredded Teflon felt. Ivalon, a synthetic polyvinyl formyl alcohol foam sponge material (Unipoint Industries, High Point, NC) has been safely used as a biologic implant for more than 30 years. This material comes packed in formalin and must be washed carefully to remove all traces of preservative. It can then be cut into small blocks and autoclaved. Before its use, Ivalon must be soaked for about 10 minutes in a saline solution to rehydrate it and allow it to become soft and pliable. A small block of the material is then carved to fit between the artery and the nerve. We usually fashion the block into a saddle-shaped structure so that it fits completely over the nerve. This structure effectively alters the arterial force vectors and creates a satisfactory decompression. In the case of a vessel inferior to the nerve, a similar type of sponge with a longer posterior element is fashioned, and this posterior element is inserted inferior to the nerve between the artery and the vein.
On several occasions, we created a sling using a partial thickness of the tentorium that was looped down around the vessel and reattached to the tentorium with a small suture. This was effective when the subarachnoid space was too cramped to place Ivalon without adding compression to the nerve but was technically much more difficult than inserting the sponge prosthesis. If venous channels alone are encountered, no prosthesis is required and the channels are coagulated and divided. Coagulation alone shrinks them, which increases the tension on the nerve and allows for potential recanalization, so the coagulated vessels should always be divided. Tumors can also be the sole cause of neural compression or found displacing a vessel against the nerve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree