INTRODUCTION
Corticobasal degeneration (CBD) was first clearly outlined in the late 1960s, originally termed “corticodentatonigral degeneration with neuronal achromasia” (1). The meticulously described cases included three individuals presenting with slowness and clumsiness in a single limb progressing to include severe rigidity and contractures, mild tremor, speech impairment, alien limb phenomenon, apraxia, eye movement limitations, and exaggerated reflexes. Cognition was described as intact apart from the presence of frontal lobe reflexes. Pathologic examinations showed frontoparietal atrophy with neuronal loss, gliosis, and distinctive achromasia (1). Subsequent cases reinforced the relationship between this characteristic movement disorder and pathology (2–4). Similar presentations, including prominent cognitive impairment, were concurrently described in Europe under the name of “Pick’s disease type 2” (5).
While these initial descriptions suggested that the movement disorder now termed “corticobasal syndrome” (CBS) was pathognomonic for underlying CBD pathology, over the last 45 years, it has become evident that the corticobasal spectrum includes a clinical syndrome (CBS) and pathologic diagnosis (CBD) which only sometimes overlap. Indeed, the clinical presentation of CBS is associated with numerous pathologic diagnoses, including various forms of frontotemporal lobar degeneration (FTLD) (6–12), progressive supranuclear palsy (PSP) (6,10,12,13), Alzheimer’s disease (AD) (7,14–16), Parkinson’s disease (PD) (9,15), Creutzfeldt–Jakob disease (CJD) (7,17), and others. Similarly, the pathologic diagnosis of CBD has various presenting phenotypes in addition to CBS, outlined further below. While focusing on CBD, this chapter will also necessarily cover issues of relevance to the clinical presentation of CBS.
VOCABULARY
Accurate vocabulary is critical in a condition with various names and a changing understanding of clinicopathologic correlations over time. In addition to the original terminology of “corticodentatonigral degeneration with neuronal achromasia” (1), early terms included “corticonigral degeneration with neuronal achromasia” (2), “corticobasal ganglionic degeneration” (18), “cortical–basal ganglionic degeneration” (19), and “corticobasal degeneration” (3). Currently, the term “corticobasal degeneration”/CBD is favored. Given evidence of the lack of reliable clinicopathologic correlation between the commonly described clinical syndrome and CBD pathology, the terms “corticobasal degeneration syndrome” and “corticobasal syndrome” are increasingly favored to distinguish the clinical presentation from the finding of CBD pathology. Currently, the abbreviation CBS is most commonly used.
Despite the movement to use more precise terminology to separate the clinical syndrome of CBS and pathologic diagnosis of CBD, this distinction is applied irregularly. Readers must be discerning when reviewing literature written prior to this clarification and newer literature which may or may not use accurate vocabulary. To meaningfully discuss CBD, terminology must be precise. This is particularly true when discussing diagnostic criteria for “CBD” (19–25), which until 2013 reflected descriptions of the clinical syndrome. These criteria continue to be widely applied and cited, but describe the CBS phenotype alone and not other potential presentations of CBD pathology. Clinical diagnosis and application of these criteria result in correct antemortem prediction of CBD pathology in only 25% to 56% of cases (7–9,13,15,26,27).
In 2013, new criteria for the clinical diagnosis of pathologic CBD were proposed (28), aiming to incorporate the understanding that CBD has multiple clinical presentations in addition to CBS. These criteria, which require further validation and revision, suggest additional new vocabulary, including “clinical research criteria for probable sporadic CBD” (cr-CBD) and “possible CBD” (p-CBD). These terms are meant to indicate that patients with these designations have clinical presentations (of which CBS is only one) felt to represent pathologic CBD, though they have not yet come to autopsy. Additionally, the criteria include terms for four clinical phenotypes associated with CBD: CBS, frontal behavioral–spatial syndrome (FBS), nonfluent/agrammatic variant primary progressive aphasia (naPPA), and progressive supranuclear palsy syndrome (PSPS). For ease of reference, terms used in discussion of the corticobasal spectrum are highlighted in Table 16.1.
PATHOLOGY
Neuropathologic CBD diagnoses are made by applying the 2002 Office of Rare Diseases criteria (29). Key features include cortical atrophy, nigral degeneration, and tau-immunoreactive lesions, particularly in the caudate and putamen (29). The ballooned achromatic neurons (Fig. 16.1A) emphasized in early pathology descriptions are a supportive but not core feature; these are now considered nonspecific, as they are also seen in other neurodegenerative conditions. Abnormal tau immunoreactivity in CBD is found in the glia and neurons in both gray and white matter. Like PSP (Chapter 15), CBD is a four-repeat (4R)-tauopathy, reflecting upregulation of the 4R-tau containing exon 10 (30,31). Although both PSP and CBD have widespread glial fibrillary tangles, CBD typically has astrocytic plaques (Fig. 16.1B), whereas PSP is characterized by tufted astrocytes, helping distinguish between the two (30). Widespread temporal lobe argyrophilic grains with a particular ultrastructure were recently suggested as a CBD-specific feature related to the underlying tauopathy (32). TAR DNA–binding protein 43 (TDP-43) pathology occurs in a subset of patients with CBD pathology (33,34), but is more common in tau-negative processes.
| Key Vocabulary Relating to Corticobasal Degeneration |
Term | Definition |
Corticobasal degeneration (CBD) | CBD is a pathologic diagnosis with abnormal aggregation of hyperphosphorylated tau. Pathologic findings also include cortical atrophy, ballooned neurons, and nigral degeneration. Historical studies use the term “corticobasal degeneration” for both clinical and pathologic diagnoses, but currently this term is reserved for pathologic diagnosis alone. |
Corticobasal syndrome (CBS) | CBS is a clinical syndrome characterized by the presence of a gradually progressive movement disorder (with features which may include parkinsonism, dystonia, and/or myoclonus) and higher cortical dysfunction (with features of apraxia, cortical sensory deficits, and/or alien limb phenomenon). When “CBS” is used in the context of the 2013 cr-CBD criteria, it requires formal application of CBS phenotype criteria (Table 16.3) and is accompanied by a “probable” or “possible” modifier. |
Clinical research criteria for probable sporadic CBD (cr-CBD) | The criteria (or diagnosis resulting from the criteria) of probable sporadic pathologic CBD made on a clinical basis using the 2013 criteria |
Frontal behavioral–spatial syndrome (FBS) | One of four accepted phenotypes in the 2013 cr-CBD criteria, FBS is a clinical syndrome that can include executive dysfunction, behavioral or personality changes, and visuospatial deficits. |
Nonfluent/agrammatic variant of primary progressive aphasia (naPPA) | One of four accepted phenotypes in the 2013 cr-CBD criteria, naPPA is a clinical syndrome involving effortful, agrammatic speech accompanied by apraxia of speech, groping, distorted speech, and/or relatively preserved single-word comprehension but impaired grammar or sentence comprehension. This term (or similar ones) is also used outside of the cr-CBD criteria in the context of recent classifications of primary progressive aphasia. |
Progressive supranuclear palsy syndrome (PSPS) | One of four accepted phenotypes in the 2013 cr-CBD criteria (for p-CBD only), PSPS includes features such as vertical supranuclear gaze palsy (or impaired vertical saccades), parkinsonism, postural instability or falls, urinary incontinence, and/or behavioral changes. This term is also used outside of the cr-CBD criteria in recent papers attempting to distinguish between clinical presentations suggestive of PSP and the pathologic diagnosis of PSP. |
Alien limb phenomena (ALP) | ALP include a variety of behaviors, including purposeless wandering of the limb, dissociation from one’s own limb, impulsive hand groping or grasping, intermanual conflict, enabling synkinesis, and magnetic apraxia. While simple levitation and parietal drift can be seen in patients with CBS and CBD, these behaviors are insufficient for a diagnosis of ALP. |
Cortical sensory loss | Cortical sensory loss refers to impairments in sensation related to cortical rather than peripheral dysfunction. Examples include Agraphesthesia (the inability to recognize writing on the skin by touch alone), which can be tested by drawing numbers on a patient’s palm Impaired stereognosis (the ability to recognize an object by touch alone), which can be tested by having a patient close his or her eyes and handing them small objects such as coins Extinction Impaired two-point discrimination |
Apraxia | Apraxia is the inability to performed skilled or learned movements (where this inability cannot be explained by other deficits). |
There are numerous reports of CBD coexisting with other pathologic diagnoses (11,35–38), with one series of PSP patients reporting coexisting CBD pathologic findings in 32% of cases (38). Given overlapping clinical presentations, shared association with the H1 tau haplotype (39), and similar 4R-tau pathologies, whether PSP and CBD may represent different presentations of a single disorder remains an area of active discussion.
CLINICAL DIAGNOSIS
Accurate in-life diagnosis of CBD is challenging due to heterogeneity of presenting phenotypes. In addition to CBS, other clinical diagnoses for patients later shown to have CBD include PSP (9–11,13,15,27,31,40–44), frontotemporal dementia (FTD) (10,13,27,31,37,40), AD (13,26,27,31,40), primary progressive aphasia (PPA)/progressive nonfluent aphasia (PNFA) and/or apraxia of speech (AOS) (13,26,37,45), and others (Table 16.2). As described above, the first criteria for the clinical diagnosis of CBD to incorporate phenotypes other than just CBS were published in 2013 (28). Application of these criteria is a two-step process (Fig. 16.2): (1) assigning a clinical phenotype or phenotypes (Table 16.3) and (2) assessing for a diagnosis of cr-CBD, p-CBD, or neither (Table 16.4).
The goal of the cr-CBD (probable sporadic CBD) criteria is to identify features that increase diagnostic specificity, that is, make it less likely that patients with cr-CBD have other diagnoses (e.g., PSP, familial tauopathy, etc.). The goal of the p-CBD criteria is to increase diagnostic sensitivity, acknowledging that the clinical presentations of p-CBD are consistent with CBD but also other pathologies (28). Few studies identify clinical characteristics that reliably predict underlying pathology in patients with these clinical phenotypes, however, limiting the diagnostic accuracy of resulting criteria. Additionally, while 8% of CBD patients are misdiagnosed clinically as having an AD-like dementia (28), an AD-like phenotype was not included in the cr-CBD diagnostic criteria given that this phenotype would dramatically increase the false-positive rate (AD being much more common than CBD). Thus, while the cr-CBD/p-CBD criteria represent an advance in that they incorporate multiple presenting phenotypes, they will require validation and continued revisions over time.
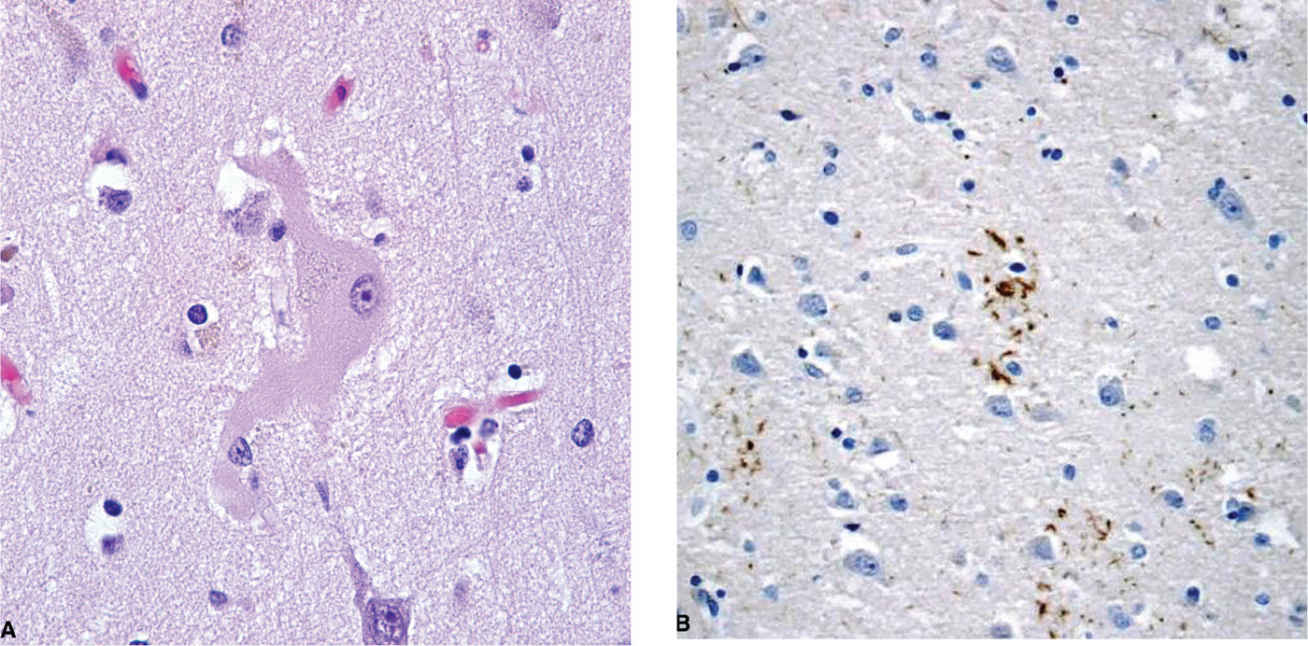
Figure 16.1. A: Ballooned neuron in a patient with corticobasal degeneration. B: Astrocytic plaque as revealed by tau immunostaining. (Courtesy of Rudolph J. Castellani, MD.)
| Clinical Diagnoses for Pathologically-Proven CBD Cases |
Diagnosis/phenotype | Initial diagnosis (%) (n = 129) | Final diagnosis (%) (n = 210) |
Corticobasal syndrome | 27.1 (35/129) | 37.1 (78/210) |
Progressive supranuclear palsy syndrome | 6.2 (8/129) | 23.3 (49/210) |
Frontotemporal dementia | 15.5 (20/129) | 13.8 (29/210) |
AD-like dementia | 9.3 (12/129) | 8.1 (17/210) |
Mixed diagnosis including these 5 phenotypes | N/A | 5.7 (12/210) |
Parkinson’s disease (PD) or atypical PD | 15.5 (20/129) | 3.8 (8/210) |
AD, Alzheimer’s disease Source: Adapted from Armstrong MJ, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80(5):496–503. | ||
One article (to date) has applied the new criteria (46), but conclusions are limited given that the cohort consisted of 10 patients all with clinically diagnosed CBS. Pathologic diagnoses included CBD (3), PSP (3), AD (3), and an atypical tauopathy (1) (46). Only one patient each met cr-CBD and p-CBD criteria within 2 years of symptom onset, both with AD pathology. While all 10 patients had CBS diagnoses, no patient met formal cr-CBD phenotypic criteria for probable CBS. The AD patient meeting criteria for cr-CBD had FBS plus possible CBS, the AD patient with p-CBD had possible CBS, and a third patient (with PSP pathology) had possible CBS but an exclusion criterion (prominent cerebellar signs).
When looking at data from any time during the disease course, 8 of the 10 patients met phenotypic criteria for possible CBS; none met criteria for probable CBS. Two of these patients (one with PSP and one with an atypical tauopathy) had exclusion criteria. Five had p-CBD diagnoses (three with CBD, one with PSP, and one with AD on autopsy) and one (with AD) had a cr-CBD diagnosis (46). This study suggests that early diagnosis is challenging, a fact well recognized by clinicians. With the appearance of additional clinical features over time, the criteria will become more sensitive. Whether they will have adequate specificity, particularly for non-CBS presentations, remains to be determined.
CLINICAL PRESENTATIONS
Of the clinical syndromes associated with CBD, CBS is clearly the best described. At its core, CBS is a clinical syndrome where patients have
1. Movement disorders features: a (usually) asymmetric akinetic rigid syndrome with dystonia, myoclonus, and/or postural instability and falls; and
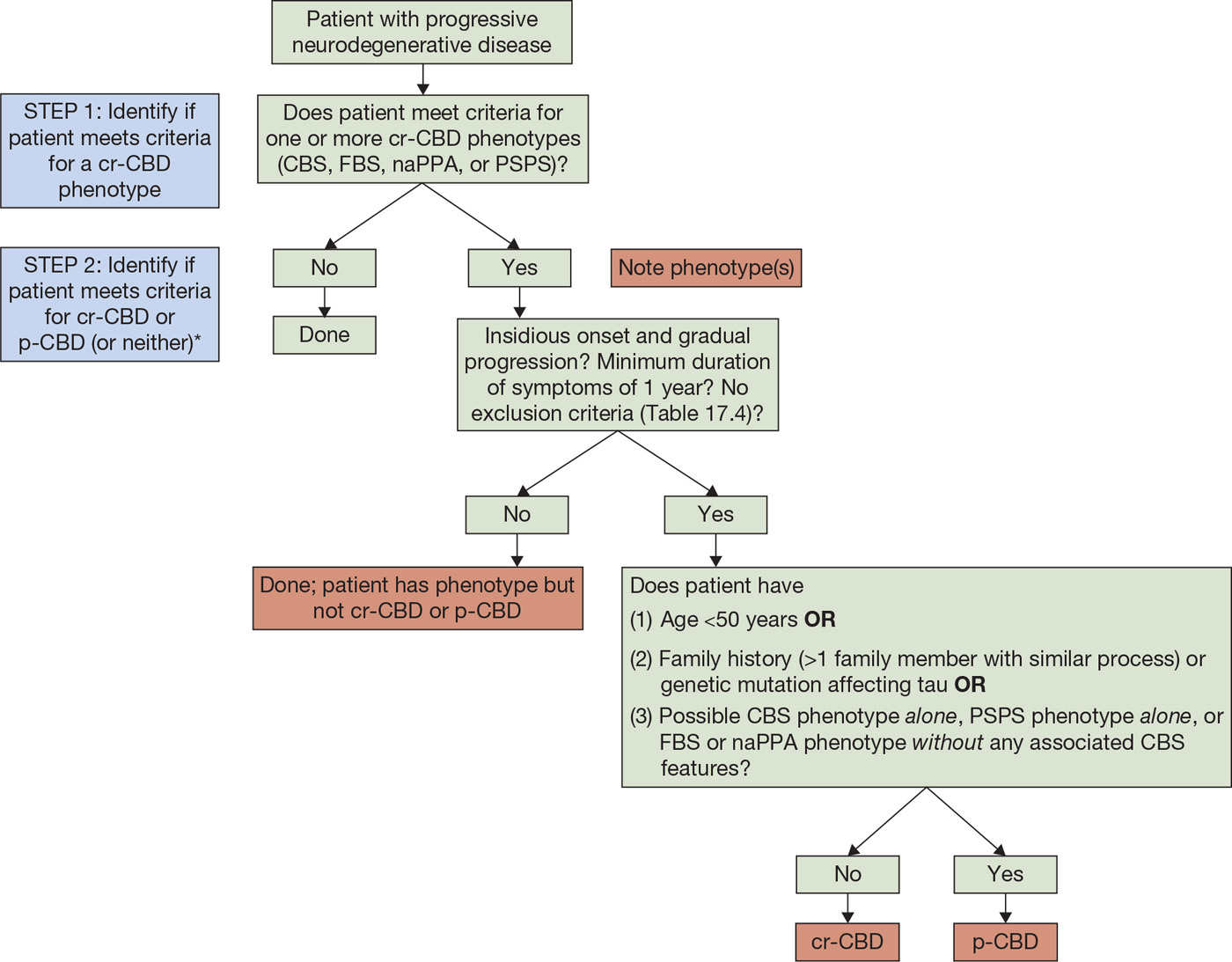
Figure 16.2. Algorithm for applying cr-CBD criteria. CBD, corticobasal degeneration; cr-CBD, clinical research criteria for CBD; p-CBD, clinical research criteria for possible CBD; CBS, corticobasal syndrome; FBS, frontal behavioral–spatial syndrome; naPPA, nonfluent/agrammatic variant of primary progressive aphasia; PSPS, progressive supranuclear palsy syndrome.
*Once a patient meets criteria for one of the phenotypes, there are three possible outcomes:
1. Patient has a phenotype (or phenotypes) but does not meet criteria for cr-CBD or p-CBD (e.g., a patient with possible CBS who has positive amyloid imaging).
2. Patient has a phenotype (or phenotypes) but only meets criteria for possible CBD (p-CBD).
3. Patient has a phenotype (or phenotypes) and meets criteria for cr-CBD.
Both the phenotype (or phenotypes) and diagnosis of cr-CBD/p-CBD (or not) should be noted.
2. Higher cortical dysfunction: cognitive–behavioral changes, aphasia, apraxia (orobuccal or limb), alien limb phenomena (more than simple levitation), and/or cortical sensory loss.
When comparing criteria formalizing this presentation, however, concordance is poor (47). Additionally, understanding how CBS reports inform our understanding of CBD presentations is challenging. Many descriptions of patients with CBS reflect cases without pathologic confirmation or series mixing cases with and without pathologic confirmation. This has resulted in a distorted view of the frequency of clinical features in CBD. For example, dystonia is present in 59% to 83% of CBS patients in four series with and without pathologic CBD diagnoses (4,19,48,49). In recent reviews of pathologically-proven CBD patients, however, the frequency of dystonia was much lower. Limb dystonia was present in only 20% of CBD cases at presentation and 38% of cases at some point in the disease course in a review utilizing brain bank patients and published cases series including at least five patients (28). In a separate review (with overlapping cases), 38% of CBD patients had dystonia (50). Myoclonus is described in 55% to 93% of patients with CBS (4,19,48,49), but only 15% of CBD patients at onset and 27% at some time in the disease course (28). Furthermore, there is research to suggest that myoclonus in CBS patients may be more suggestive of underlying AD pathology than CBD pathology (14,46).
Patients with CBS classically present with an asymmetric akinetic rigid syndrome. In a predominantly clinical series, 95% of patients had at least two parkinsonian features (bradykinesia, rigidity, tremor, and gait disorder) (48). Limb rigidity and bradykinesia are present at presentation in approximately half of CBD cases, increasing to over 75% over time, with 73% of CBD patients having documented asymmetry (28). Abnormal gait, postural instability, and falls in CBD patients also increase over time, from 33% to 41% at onset to 73% to 78% prior to death. Tremor is reported in CBS and CBD, but the phenotype is poorly characterized and repetitive myoclonic bursts may be mistaken for tremor (28). As mentioned above, dystonia is more common in CBS than CBD. Dystonia in both CBS (49) and CBD (50) most commonly affects the upper extremities, though lower limb dystonia is described (49,50). Myoclonus usually co-occurs with dystonia: 64% of CBD patients with dystonia have myoclonus, whereas only 4% of patients have myoclonus in the absence of dystonia (50). In general, the movement disorder in both CBS and CBD is poorly responsive to treatment, with a sustained levodopa response serving as an exclusion criterion in both CBS (20,21,24,25) and cr-CBD/p-CBD criteria (28).
| Criteria for cr-CBD Phenotypes |
Phenotype | Criteria |
Probable corticobasal syndrome (CBS) | Asymmetric presentation of at least two of the following: • Limb rigidity and/or akinesia • Limb dystonia • Limb myoclonus and at least two of the following: • Orobuccal or limb apraxia Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




