Cranial Nerve I (The Olfactory Nerve)
Anatomy of the Olfactory Pathways
Although the olfactory system is not of major importance in neurologic diagnosis, certain clinical information, useful in neuroanatomic localization, may be attained by investigating the sense of smell. This investigation requires a basic knowledge of the anatomy of the olfactory pathways [3,6,9,30,37], especially their relationship with the surrounding neural structures (e.g., frontal lobes) (Figs. 6.1 and 6.2).
The olfactory receptors, the sensory cells of the olfactory epithelium, are located on the superior– posterior nasal septum and the lateral wall of the nasal cavity. These ciliated cells give off central processes that form small bundles (approximately 20 in number). These bundles, the filaments of the olfactory nerve, penetrate the cribriform plate of the ethmoid bone and enter the olfactory bulb. Here, the olfactory afferent fibers synapse with the dendrites of the second-order neurons called the mitral and tufted cells. At the points of synapse, conglomerates of fibers called the olfactory glomeruli are formed. The axons of the mitral and tufted cells leave the olfactory bulb and course posteriorly, as the olfactory tract, in the olfactory sulcus on the orbital surfaces of the frontal lobe. The olfactory tract divides into a median and a lateral olfactory stria on either side of the anterior perforated substance (the triangular area formed by the two striae is called the olfactory trigone). Some of these strial fibers decussate in the anterior commissure and join the fibers from the opposite olfactory pathways, terminating in the contralateral cerebral hemisphere. Other strial fibers, especially those of the lateral stria, supply the ipsilateral piriform lobe of the cerebral (temporal) cortex (the primary olfactory cortex) and terminate in the amygdaloid nucleus, septal nuclei, and hypothalamus.
Although relatively quantitative methods [6,30,37] are available to test olfaction (e.g., tests of the minimal perceptible odor or measurements of olfactory fatigue), the sense of smell is usually tested by asking the patient to sniff various nonirritating substances (each nostril is tested separately) and then attempt to identify the odor (perception of the smell is of more value than identification of the specific substance). Irritating substances (e.g., ammonia) are to be avoided because they stimulate the trigeminal nerve fibers in the nasal mucosa as well as the olfactory fibers.
Localization of Lesions Affecting the Olfactory Nerve
Lesions Causing Anosmia
Anosmia (loss of smell) or hyposmia (diminished olfactory functioning) may or may not be apparent to the patient. He or she may have some difficulty in tasting various flavors because the identification of tasted substances depends in part on the olfactory system.
Decreased smell function occurs in the “normal” elderly [12]. Generally, age-related decline in olfaction is more severe for men than for women. Approximately 2% of the population younger than 65 years has a chronic impairment of smelling. Between 65 and 80 years, this rises dramatically with about half of the population experiencing significant decrements in the ability to smell. For the population older than 80 years, this figure rises to nearly 75% [12]. The basis for age-related changes in smell function is multiple and includes ossification and closure of the foramina of the cribriform plate, development of early neurodegenerative disease pathology, and cumulative damage to the olfactory receptors from repeated viral and other insults throughout life [8].
Local nasal disease (e.g., allergic rhinitis, nasal obstruction, polyposis) must first be sought as the cause of anosmia, especially if the olfactory difficulty is bilateral. The most common cause of transient and bilateral anosmia is the common cold. Exposure to several airborne toxins, including herbicides, pesticides, solvents, and heavy metals can alter the ability to smell, especially when exposure has been chronic. Among the heavy metals, the best documented cases are for cadmium, chromium, nickel, and manganese [8].
After local nasal disease has been ruled out, anosmia, especially unilateral anosmia, should raise the suspicion of a lesion affecting the olfactory nerve filaments, bulb, tract, or stria. Because the cortical representation for smell in the piriform cortex is bilateral, a unilateral lesion distal to the decussation of the olfactory fibers causes no olfactory impairment.
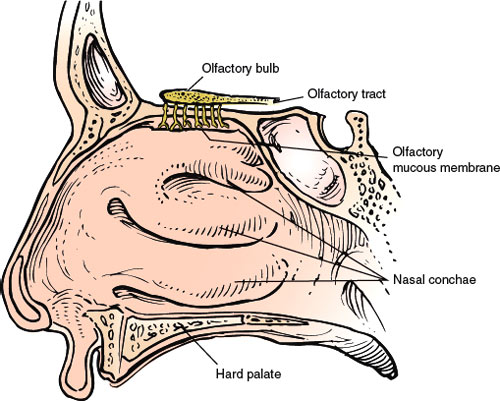
FIG. 6.1. The olfactory nerve (lateral view).
Head injury [13,36,41] is probably the most common cause of disruption of the olfactory fibers prior to their decussation. Frontal impact produces less dysfunction than back or side impacts [13]. The olfactory nerve proper (olfactory filaments) may be torn by fractures involving the cribriform plate of the ethmoid bone, but closed head injury without fracture may also disrupt the olfactory pathways unilaterally or bilaterally. Closed head injury can produce impairment of olfactory recognition despite relatively preserved olfactory detection [25]. Olfactory naming and recognition may be impaired by traumatic forces affecting the orbitofrontal and temporal lobes, and the degree of olfactory disturbances is directly related to the severity of the injury [25]. Disturbances of complex olfactory function (e.g., discrimination) despite the relatively preserved detection of odors have been reported with alcoholic Korsakoff syndrome [23] and following thalamic or prefrontal cortical lesions [34]. Significant olfactory dysfunction has also been described with Alzheimer disease [27,28,46], Lewy body disease [32], Huntington chorea (HC) [28], corticobasal ganglionic degeneration [33], Creutzfeldt–Jakob disease [42,52], frontotemporal dementia [33], multiple sclerosis [10,20], Parkinson disease (PD) [11,21,27,43], Refsum disease [17], spinocerebellar ataxias (including Friedreich ataxia) [4], Wilson disease [31], narcolepsy [39], pure autonomic failure [38], and in adults with Down syndrome [45]. Impaired olfaction can predate clinical PD in men by at least 4 years and may be a useful screening tool to detect those at high risk for development of PD in later life [48]. Patients with PD have decreased performance on odor discrimination tests in addition to deficits of odor detection and identification [43]. REM (rapid eye movement) sleep disorder and olfactory dysfunction are common and very early features of alpha-synucleinopathies, in particular, PD [40]. Olfactory loss in patients with multiple sclerosis has been associated with plaque formation in the central olfactory (i.e., inferior frontal and temporal) brain regions [10].
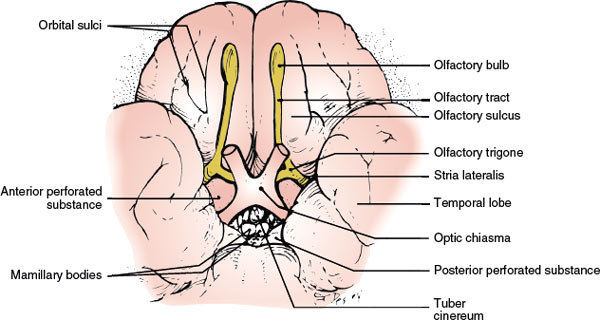
FIG. 6.2. The olfactory nerve (inferior view).
Hawkes [19] noted that there has been an increase of interest in olfactory dysfunction because it was realized that anosmia was a common feature of idiopathic PD and Alzheimer- dementia (AD). In his review of PD, parkinsonian syndromes, essential tremor, AD, motor neuron disease, and HC, the following observations are made [19]:
1. Olfactory dysfunction is frequent and often severe in PD and AD.
2. Normal smell identification in PD is rare and should prompt the review of diagnosis unless the patient is a female with a tremor-dominant disease.
3. Anosmia in suspected progressive supranuclear palsy and corticobasal degeneration is atypical and should likewise provoke diagnostic review.
4. Hyposmia is an early feature of PD and AD and may precede motor and cognitive signs, respectively.
5. Subjects with anosmia and one apoE4 allele have an approximate fivefold increased risk of later AD.
6. Impaired sense of smell is seen in some patients at 50% risk of parkinsonism.
7. Smell testing in HC and motor neuron disease, where abnormality may be found, is not likely to be of clinical value.
8. Biopsy of olfactory nasal neurons shows nonspecific changes in PD and AD and, at present, will not aid diagnosis.
Incidental Lewy bodies (ILB), the presence of Lewy bodies in the brains of deceased individuals without a history of PD or dementia during life, are thought to represent a presymptomatic stage of PD. Olfactory dysfunction is associated with ILB [35]. If ILB represent a presymptomatic stage of PD, olfactory testing may be a useful screening tool to identify those at high risk for developing PD.
Congenital anosmia or hyposmia may occur owing to cleft palate in men, absent or hypoplastic olfactory bulbs or tracts, familial dysautonomia, and Turner syndrome. A familial syndrome of permanent anosmia with hypogonadotropic hypogonadism (Kallmann syndrome) has also been described [50,51]; patients with this syndrome may also have cerebellar ataxia and mirror movements of the hands [18].
Olfactory discrimination and detection may be abnormal after unilateral frontal or temporal lobectomy [53]. After temporal lobectomy, deficits in olfactory discrimination are confined to the nostril ipsilateral to the lesion. After frontal lobectomy, discrimination is also impaired; however, in patients with right frontal lesions including the orbital cortex, the impairment is found in both nostrils. Therefore, the orbitofrontal cortex is important in olfactory discrimination, and the nostril difference found in healthy subjects, together with the birhinal impairment in patients with right orbitofrontal damage, suggests a relative advantage of the right orbital region in olfactory processing [53]. Anosmia may also complicate rhinoplasty, ethmoidectomy, laryngectomy, submucous resection of the nasal septum, radiotherapy [30], and surgery for anterior communicating artery aneurysms owing to the olfactory nerve dysfunction [15]. Olfactory damage is much more common after an anterior interhemispheric surgical approach rather than after a basal interhemispheric approach.
The olfactory bulb and tract are frequently affected by tumors of the olfactory groove (especially meningiomas) [49], which may cause the Foster Kennedy syndrome (see the discussion on Foster Kennedy syndrome in the next section). Tumors of the sphenoid or frontal bone (e.g., osteomas), pituitary tumors with suprasellar extension, nasopharyngeal carcinoma [44], and saccular aneurysms of the anterior portion of the circle of Willis (e.g., a giant anterior communicating artery aneurysm) may also compress the olfactory bulb or tract [26]. Any diffuse meningeal process (e.g., meningitis) may involve the olfactory pathways. The anatomic relationship of the frontal lobe to the olfactory bulb and tract is especially important. Mass lesions of the frontal lobe (e.g., glioma or abscess) often exert pressure on the olfactory system and may lead to anosmia even before clear-cut frontal lobe signs and symptoms are noted. Therefore, in any patient with personality changes or subtle signs of frontal lobe involvement, olfaction should be carefully tested.
TABLE 6.1 Conditions Associated with Disturbance of Olfaction










