Cranial Nerve VIII (The Vestibulocochlear Nerve)
Anatomy of Cranial Nerve VIII
The eighth cranial nerve consists of two separate functional components: the auditory (cochlear) nerve concerned with hearing and the vestibular nerve concerned with equilibrium. Phylogenetically, the vestibular system antedates the cochlear system [40]. The auditory nerve receives information from the tonotopically organized cochlea, the organ of hearing. The vestibular nerve derives its input from the saccular and utricular macules (which sense linear acceleration) and the cristae of the semicircular canals (which sense angular acceleration of the head). Because of this functional dualism, the two vestibulocochlear nerve components are discussed separately.
The Auditory Pathways
The auditory pathways (Fig. 11.1) may be conceptualized as a four-tiered neuronal network, as follows: (a) auditory (cochlear) nerve extending from the organ of Corti to the cochlear nucleus, (b) fibers from the cochlear nucleus crossing to the contralateral inferior colliculus, (c) fibers from the inferior colliculus extending to the medial geniculate body, and (d) fibers from the medial geniculate body projecting to the auditory cortex in the superior temporal gyrus [25,73,130].
FIRST-ORDER NEURONS
The auditory receptors are the neuroepithelial hair cells of the organ of Corti. The structure of the cochlea is such that hair cells located at the cochlear apex are stimulated by low-frequency tones, whereas those located at the base are stimulated by high-frequency tones. First-order neurons of the auditory pathway have their cell bodies in the spiral ganglion of the cochlear nerve, which lies in Rosenthal’s canal at the base of the bony spiral lamina. Afferent components of these cells make contact with the hair cells of the cochlea, the majority converging on the inner hair cells and a smaller number diverging to make contact with the outer hair cells. When the hair cells are activated, impulse transmission is triggered in fibers having their perikarya in the spiral ganglion; these fibers then enter the brainstem, at the level of the ventral cochlear nuclei, as the cochlear nerve.
SECOND-ORDER NEURONS
On entry into the lower brainstem at the junction between the medulla and pons, the afferent cochlear nerve fibers divide, innervating the dorsal cochlear nucleus and the anteroventral and posteroventral nuclei of the cochlear complex. This innervation follows a tonotopic pattern. The more dorsal aspects of these nuclei receive fibers that have innervated “high-frequency” (basal) hair cells, whereas the ventral aspects receive fibers from “low-frequency” (apical) hair cells. The dorsal and ventral cochlear nuclei contain the second-order neurons and give rise to several projections to the contralateral brainstem, which ascend as the lateral lemniscus, a fiber tract that projects to the central nucleus of the inferior colliculus. These projections include the dorsal acoustic striae (from the dorsal cochlear nucleus), the intermediate acoustic striae (from the dorsal part of the ventral cochlear nucleus), and the ventral acoustic striae (from the ventral cochlear nucleus), which is part of the trapezoid body. Decussating fibers of the trapezoid body and in the contralateral superior olivary complex ascend in the contralateral lateral lemniscus. The lateral lemniscal fibers ascend, with some fibers terminating in the lateral lemniscal nuclei along the way, and terminate in the inferior colliculus. (The ventral acoustic striae also terminate in the ipsilateral and contralateral reticular formation, the superior olivary nuclei, and the nuclei of the trapezoid body.)
THIRD-ORDER NEURONS
The inferior colliculus, located in the midbrain tectum caudal to the superior colliculus, contains the third-order neurons and serves as the central relay nucleus in the auditory pathway, receiving ascending and descending input. Basically, all ascending auditory pathways end in the inferior colliculus. Fibers from the lateral lemniscus end in the prominent central nucleus of the inferior colliculus, which has a tonotopic organization. The projections from the inferior colliculus terminate in the medial geniculate body, with the low-frequency fibers ending in the apical–lateral areas and the high-frequency fibers ending in the medial portions of this nuclear mass.
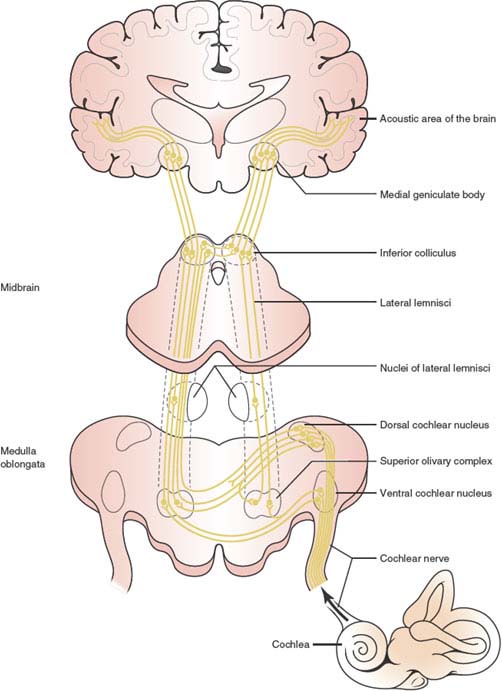
FIG. 11.1. The auditory pathways. (Adapted from Stockard JJ, Stockard JE, Sharbrough FW. Detection and localization of occult lesions with brainstem auditory responses. Mayo Clin Proc 1977;52:761–769.)
FOURTH-ORDER NEURONS
The medial geniculate body is the thalamic auditory relay nucleus, and chiefly gives rise to the geniculotemporal fibers or auditory radiations. The auditory radiations course laterally in a dense tract that partly penetrates the ventral and lateral portions of the posterior half of the putamen and partly runs in the white matter below it [162]. Most fibers terminate in lamina IV of the primary auditory cortex (AI, Brodmann’s area 41), located in the transverse temporal gyri of Heschl, but some end in the association auditory cortex (AII, Brodmann’s area 42). The primary auditory cortex terminations conform to a tonotopic pattern, with high-frequency tones terminating medially and low-frequency tones terminating laterally. Brodmann’s area 41 is reciprocally connected with the ventral division and Brodmann’s area 42 with the dorsal division of the medial geniculate body. Through a large fiber tract of the corpus callosum, each auditory cortical area is likewise connected with the reciprocal areas in the other cerebral hemisphere.
There are many commissural connections along the auditory pathway; however, none exists at the level of the medial geniculate body. The bilaterality of representation has obvious significance when unilateral brainstem lesions are considered. The neurons of the superior olivary complex receive input from both ears. There are connections between the two cochlear nuclei, connections between the two dorsal nuclei of the lateral lemniscus through the commissure of Probst, connections between the inferior colliculus on each side through the commissure of the inferior colliculus, and connections between the central nucleus of the inferior colliculus and the contralateral medial geniculate body through the brachium of the inferior colliculus [119]. Another important consideration is that from the inferior colliculus upward, there are two different projection systems. The first (including the central nucleus of the inferior colliculus, portions of the medial geniculate, and the primary auditory cortex) is referred to as a core system, which is a direct auditory pathway with a tonotopic organization. The other (including the pericentral region of the inferior colliculus, the nonlaminated portions of the medial geniculate body, and the secondary auditory cortex) is referred to as a belt projection, which has less tonotopic organization and serves as a polymodal system that receives both auditory and nonauditory information.
There are also several descending auditory pathways that run parallel to the ascending fibers and are integrated in the feedback control of auditory input. They include corticogeniculate fibers, corticocollicular fibers, geniculo-collicular fibers, collicular efferents, and an efferent cochlear bundle from the superior olivary complex to the hair cells of the spiral organ of Corti.
The blood supply to the cochlea and auditory brainstem nuclei arises from the internal auditory (labyrinthine) artery, usually a branch of the anterior inferior cerebellar artery. Within the internal auditory canal, the internal auditory artery supplies the ganglion cells, nerves, dura, and arachnoid membranes, and then divides into the common cochlear artery and the anterior vestibular artery. The superior olivary complex and lateral lemniscus are supplied by circumferential branches of the basilar artery, the inferior colliculus is vascularized by branches of the superior cerebellar and quadrigeminal arteries, and the medial geniculate bodies receive their blood supply from the thalamogeniculate arteries. Branches of the middle cerebral artery supply the primary auditory and associated cortices.
The Vestibular System
The vestibular system monitors angular and linear accelerations of the head. This information is used to monitor the motion and position of the head in space and to maintain balance. These accelerations are transduced into neuronal signals within a specialized structure, the membranous labyrinth. The labyrinth consists of the otolith organ (utricle and saccule) and the three semicircular canals [3,26,41]. Linear acceleration is monitored by specialized receptors, the macules, of the utricle and saccule, whereas angular acceleration is monitored by the cristae in the ampullae of the semicircular canals. These receptors are composed of numerous hair cells that serve as transducers, converting mechanical movements of sensory hairs into changes of receptor potentials in the hair cells and in their afferent neurons.
The semicircular canals are three in number and are oriented at approximately right angles to each other to detect angular accelerating movements of the head. These canals include the lateral or horizontal canal (with an outward convexity), the anterior or superior canal (with an upward convexity), and the posterior or inferior canal (with a backward convexity). When the head is in the erect position, the horizontal canal is almost horizontal (there is a slight inclination down and back, forming a 30-degree angle with the horizontal), and the superior and posterior canals are arranged in two vertical planes that form a 45-degree angle with the frontal and sagittal planes. Therefore, the horizontal canals of both labyrinths are in the same plane, whereas the superior canal on one side is in the same plane as the posterior canal of the opposite side.
The utricle and saccule are arranged at right angles also, with the utricle parallel to the base of the skull and the saccule parallel to the sagittal plane [63]. Therefore, horizontal head movements stimulate the utricle linearly, whereas tilting the head activates the saccule.
When the cristae or macules are stimulated, potentials are developed in their afferent nerve endings, the cell bodies of which lie in the vestibular ganglion of Scarpa housed in the internal acoustic meatus. These impulses are then transmitted through the nerve fibers that make up the vestibular nerve.
The information from the membranous labyrinth is transmitted in a different manner in the two different components of the vestibular nerve. The superior portion of the nerve carries input from the anterior and horizontal semicircular canals and from the utricle, whereas the inferior portion of the nerve transmits information from the posterior semicircular canal and the saccule. The vestibular nerve enters the brainstem at the pontomedullary level, bifurcates into ascending and descending fascicles, and terminates in the vestibular nuclei (the superior nucleus of Bechterew, the lateral nucleus of Deiters, the medial nucleus of Schwalbe, and the inferior or descending nucleus of Roller), which lie in the rostral medulla and caudal pons. The vestibular nuclei initiate contralateral vestibulo-ocular responses and ipsilateral vestibulospinal reflexes to maintain a stable vision during head movements and a stable posture during body movements [81,144]. The semicircular canals relate preferentially to the superior and medial vestibular nuclei, whereas the macular fibers project mainly to the medial and inferior vestibular nuclei. Other afferents of the vestibular nerve enter the cerebellum by way of the inferior cerebellar peduncle and terminate in the vestibulocerebellum.
Most of the vestibular nuclei output is concerned with feedback integration with the cerebellum, spinal cord, and brainstem. The main vestibular connections include the following structures.
MEDIAL LONGITUDINAL FASCICULUS
Through the medial longitudinal fasciculus (MLF), the vestibular nuclei exert an influence on conjugate eye movements and on head posture. Although all the vestibular nuclei make contributions to the MLF, only the superior nucleus projects to the ipsilateral MLF; other nuclei send fibers to the contralateral MLF.
MEDIAL VESTIBULOSPINAL TRACT
The medial vestibulospinal tract arises primarily from the medial vestibular nucleus, and to a lesser extent, from the inferior and lateral vestibular nuclei. Through this tract the medial vestibular nucleus exerts an excitatory and inhibitory effect on the cervical and upper thoracic levels of the contralateral spinal cord.
LATERAL VESTIBULOSPINAL TRACT
This pathway originates primarily from the lateral and inferior vestibular nuclei and projects to the ipsilateral spinal cord. The fibers destined for the cervical cord arise from the rostroventral portion of the lateral vestibular nucleus, whereas the lumbosacral fibers originate from the dorsocaudal portion. The lateral vestibulospinal pathway facilitates extensor trunk tone and the action of antigravity axial muscles, reflecting the input the vestibular nucleus receives from the utricular “gravity detector.”
CEREBELLUM
The vestibulocerebellum receives afferent fibers from the vestibular ganglion and from the vestibular nuclei of the same side. The vestibular nuclei (primarily the inferior and medial nuclei) project to the ipsilateral flocculonodular lobe and uvula and to the fastigial nucleus. The cerebellum also has reciprocal connections with the vestibular nuclei including cerebellar corticovestibular fibers from the nodulus, uvula, flocculus, and other areas of the cerebellar vermis, and fastigiovestibular fibers projecting from the fastigial nucleus. All of these connections course through the juxta-restiform body.
RETICULAR FORMATION
Through its cerebellar projections, the vestibular nuclei influence the reticular formation (especially the lateral reticular nucleus and the nucleus reticularis pontis caudalis). The vestibular nuclei also project fibers back to the hair cells of the membranous labyrinth. These fibers probably serve a modulating function.
Neurons in the superior lateral and inferior vestibular nuclei project bilaterally to the ventral posterolateral and posterior nuclear group of the thalamus. The cortical representation of vestibular function is located in the postcentral gyrus near areas 2 and 5 of the cerebral cortex. Other receptive areas include the frontal lobe (area 6) and the superior temporal gyrus.
The blood supply to the membranous labyrinth is from the internal auditory or labyrinthine artery [114]. The latter usually arises from the anterior inferior cerebellar artery but occasionally branches directly from the basilar artery. After giving off a branch to the eighth nerve in the cerebellopontine angle, the internal auditory artery transverses the internal auditory meatus and, at the labyrinth, branches into (a) the anterior vestibular artery to the anterior and lateral semicircular canals and the utricular macula, (b) the posterior vestibular artery to the posterior semicircular canal, the saccular macula, and part of the cochlea, and (c) the cochlear artery. Therefore, the anterior and posterior vestibular arteries supply structures innervated by the superior and inferior branches of the vestibular nerve, respectively.
Clinical Evaluation of Cranial Nerve VIII Function
Early diagnosis of deafness is critical, as it may lead to learning disabilities and impaired language skills. According to severity, hearing loss is classified as mild (20–39dB), moderate (40–69 dB), severe (70–89 dB), or profound (>90 dB). Based on age at onset, hearing loss is categorized as prelingual or postlingual [142,152].
Sensorineural Deafness
Sensorineural deafness refers to a deficit in perceiving either tones or speech, which is due to a lesion central to the oval window. It may therefore involve the cochlea (sensory), the cochlear nerve and nuclei (neural), or the central auditory pathways. Sensorineural deafness may be bilateral and progressive (e.g., presbyacusis, ototoxic drugs), unilateral and progressive (e.g., Ménière’s disease, acoustic neuroma), or unilateral and sudden (e.g., impaired cochlear blood flow, viral infection, perilymphatic fistula, autoimmune inner ear disease or, rarely, acoustic neuroma) [105,130].
Individuals suffering from sensorineural hearing loss frequently have difficulty hearing high-pitched sounds and vowels (e and i to a greater extent than a, o, or u). Formal audiometric testing usually reveals a loss of speech discrimination that is out of proportion to associated pure tone deafness [46]. Patients with sensorineural pathology often complain of tinnitus, which varies in both pitch and intensity.
The evaluation of hearing loss begins with a thorough examination of the external auditory canal and an inspection of the tympanic membranes through otoscopy. Examination should include a detailed general physical and neurologic examination, including a thorough search for any craniofacial, musculoskeletal, ocular, hair, or skin pigmentary abnormalities. Next, it must be determined whether the hearing loss is sensorineural, conductive (i.e., lesion located between the environment and the organ of Corti), or mixed. Hearing loss may be due to a heterogeneous group of genetic (syndromic and nonsyndromic) disorders, environmental, infectious, inflammatory, autoimmune, vasculitic, metabolic, traumatic, and structural conditions, as well as selective cochlear neurotoxicity. Approximately 25% cases of childhood hearing impairment in the United States are attributable to factors such as prematurity, congenital hyperbilirubinemia, neonatal hypoxia, bacterial meningitis, viral infections (rubella, measles, herpes, cytomegalovirus, trauma, and ototoxic medications) [152]. Genetic hearing loss can follow an autosomal dominant, autosomal recessive, X-linked, or mitochondrial inheritance pattern. Most cases are nonsyndromic and autosomal recessive [47]. Drug-induced cochlear toxicity is a well-known complication of aminoglycoside antibiotics, loop diuretics, nonsteroidal anti-inflammatory drugs, quinine, and salicylates. At higher doses, aspirin can cause tinnitus, dizziness, and hearing loss. Hearing loss has also been reported in association with the use of high doses of propoxyphene and hydrocodone/acetaminophen (Vicodin) [53,66,74,136]. Reversible hearing loss related to gabapentin may occur in patients with preexisting renal dysfunction [143].
Progressive bilateral sensorineuro hearing loss is a cardinal feature of superficial siderosis of the central nervous system [94]. Progressive sensorineuro hearing loss may also be secondary to leptomeningeal carcinomatosis [127]. Hearing loss is also a common complication of cranial radiation. Hearing loss and tinnitus have also been reported with the use of Vinka alkaloids. Ototoxicity with high-frequency sensorineural hearing loss is seen with the use of cis-platinum; previous or concomitant canal radiation may lead to enhanced cis-platinum ototoxicity. Hearing loss has also been reported with the use of interferon alpha [85,109,123,151]. Progressive unilateral or bilateral hearing loss has also been described with Fabry’s disease, an inherited disorder of glycosphingolipid metabolism due to a deficiency of alpha-galactosidase activity [168]. A possible association between antiphospholipid antibodies and sensorineural hearing loss has also been proposed [125].
Common causes of conductive hearing loss are obstructions of the external auditory meatus by wax, otosclerosis, tympanosclerosis, and various external and middle ear diseases (otitis externa, serous otitis media, trauma to the ossicular chain or cholesteatoma).
In bedside qualitative assessment of hearing loss, a tuning fork (256 or preferably 512 Hz) is used to distinguish between these two types of hearing loss. Later, more formal quantitative audiologic tests are performed. Three major tuning fork tests are used for the evaluation of hearing loss: Weber’s, Rinne’s, and Schwabach’s tests.
THE WEBER’S TEST
The purpose of the Weber’s test is to help differentiate a conductive hearing loss from a sensorineural hearing loss in a unilateral hearing loss. This test is conducted by placing a vibrating tuning fork over the midline of the skull or forehead, over the nasal bone, or over the anterior upper incisors. Normally, the vibrations are perceived equally in both ears (no lateralization) because bone conduction is equal bilaterally. In conductive hearing loss, the vibrations are louder in the deaf ear (lateralized to the diseased ear). In sensorineural hearing loss, the sound is louder in the normal ear (lateralized to the normal ear).
THE RINNE’S TEST
The Rinne’s test compares the patient’s air and bone conduction. The stem of the vibrating tuning fork is applied against the mastoid process. When the patient no longer hears the vibration, the fork is placed next to the ear (approximately 1 cm from the external auditory meatus) with the tines parallel to the sagittal plane of the skull, 1 to 2 inches from the external auditory meatus. In normal individuals, because air conduction is better than bone conduction, the vibrations are perceived in the ear after they are no longer perceived at the mastoid. The Rinne’s test result is said to be normal, or positive, when the tuning fork is heard approximately twice as long by air conduction as by bone conduction. In cases of conduction deafness, bone conduction is better than air conduction, and therefore the tuning fork cannot be heard when it is placed next to the ear. With sensorineural hearing loss, both air and bone conduction are diminished to a similar extent, and air conduction remains greater than bone conduction.
THE SCHWABACH’S TEST
As in the Rinne’s test, the tuning fork is held against the mastoid process until the patient is unable to perceive any sound. The examiner then places the tuning fork over his or her mastoid bone and compares the bone conduction to that of the patient. If the examiner hears the tuning fork after the patient no longer hears it, a sensorineural hearing loss is suspected.
In summary, in sensorineural hearing loss:
1. The Weber’s test lateralizes to the normal ear.
2. The Rinne’s test result is positive (air conduction is better than bone conduction).
3. The Schwabach’s test demonstrates that the patient’s bone conduction is worse than the examiner’s.
In conductive hearing loss:
1. The Weber’s test lateralizes to the diseased ear.
2. The Rinne’s test result is negative (bone conduction is better than air conduction).
3. The Schwabach’s test is normal or prolonged (the patient may hear the tuning fork longer than the examiner does).
Vertigo and Vestibular Function
The clinical evaluation of a patient affected with dizziness or vertigo should focus on the following four areas [24,64,164].
DEFINITION OF CHARACTERISTICS OF SYMPTOMS
Dizziness is a nonspecific term often meaning different things to people (e.g., lightheadedness, head swimming, faintness or presyncope, disequilibrium, disturbance of consciousness, and true vertigo). Vertigo is an illusion of motion that some interpret as subjective (patient feels that he or she is spinning) or objective (the environment seems to be spinning). The most common illusion is a spinning or whirling sensation. Vegetative symptoms, such as nausea, vomiting, pallor, and sweating, are frequently present in patients with vertigo. Vertigo arises from an imbalance of the vestibular tone and is usually associated with disease of the labyrinth or its central connections. The history and physical examination should help distinguish peripheral from central causes of vertigo. Nausea and vomiting are much more common and severe when vertigo results from affection of the peripheral vestibular apparatus.
Vertigo must be differentiated from the following:
1. Lightheadedness or presyncopal faintness, which is caused by decreased blood flow to the brain.
2. Disequilibrium, which is characterized by an imbalance or unsteadiness while standing or walking caused by loss of vestibulospinal, proprioceptive, visual, or motor integration.
3. Nonspecific, vague symptoms of floating, swimming, giddiness, rocking, falling, and spinning inside the head. This is often due to psychogenic or psychiatric disorders causing dizziness and may be seen among patients with anxiety disorder (panic attacks, agoraphobia, obsessive-compulsive disorder), somatoform disorders (including conversion disorder), and depression. Hyperventilation may also cause dizziness.
ASSOCIATED AUDITORY SYMPTOMS
If unilateral hearing loss accompanies vertigo, primary ear disease should be suspected. A feeling of “fullness” in the affected ear may occur with external, middle, or inner ear pathology. Autophony, or the perception of the reverberation of the patient’s own voice in the affected ear, occurs only with external or middle ear disease.
Tinnitus is a sensation of noise in one or both ears in the absence of any significant stimulus (i.e., any perceived noise not produced by external auditory stimuli). Tinnitus is usually described as hissing, humming, whistling, or ringing. Tinnitus may be pulsatile (coinciding with the patient’s heartbeat) or continuous (nonpulsatile). Tinnitus may be subjective and only perceived by the patient, or objective and thus perceived by another person [70,106,135]. Tinnitus may occur in the context of “normal” hearing, but may be associated with vestibular schwannomas, presbyacusis, osteosclerosis, temporal bone trauma, temporal bone surgery, Ménière syndrome, chronic noise trauma, acute acoustic trauma, ototoxicity, vestibulotoxicity, and sudden hearing loss [39,106]. Tinnitus may be paroxysmal or continuous, pulsatile or nonpulsatile, and is more frequently noted with peripheral rather than with central lesions. Patients with unilateral tinnitus, pulsatile tinnitus, fluctuating tinnitus, or tinnitus associated with vertigo must be thoroughly assessed for potentially severe underlying pathologic processes [110]. Low roaring tinnitus suggests Ménière’s disease, whereas high-pitched tinnitus suggests presbyacusis or an acoustic tumor. Pulsatile tinnitus is usually a subjective appreciation of the patient’s normal heartbeat, but may also occur with various neoplastic and congenital or acquired arterial or venous disorders including glomus (paragangliomas), tumors, hemangiomas, meningiomas, arterial tortuosity, vascular loops, persistent stapedial artery, “aberrant” carotid artery (hypertrophic inferior tympanic artery), high-grade carotid artery stenosis, cervicocephalic arterial dissections, fibromuscular dysplasia of the internal carotid artery, intracranial aneurysms, intracranial arteriovenous malformations, intracranial or extracranial dural arteriovenous fistulae, high jugular bulb, jugular diverticulum, dehiscent jugular bulb, enlarged jugular vein, and venous stenosis. Paragangliomas most likely to present with pulsatile tinnitus are the glomus jugulare tumor, the glomus tympanicum tumor, and the glomus jugulotymapnicum tumor [177]. Pulsatile tinnitus may also occur with intracranial hypertension associated with aqueductal stenosis [155] and idiopathic intracranial hypertension [80,117,141,178]. Tinnitus from idiopathic intracranial hypertension is thought to be due to turbulence of blood flow from the hypertensive intracranial circulation into the low-pressure jugular bulb [117]. Unilateral venous hum tinnitus may be secondary to turbulent flow in the internal jugular vein [134].
Gaze-evoked tinnitus may develop after removal of cerebellopontine angle tumors (e.g., acoustic neuroma) [176]. This type of tinnitus may be associated with saccades, pursuit, and vestibulo-ocular eye movements. It is postulated to be due to an abnormal interaction between the vestibular and cochlear nuclei, possibly due to neural sprouting after transection of the auditory nerve [176]. Other causes of tinnitus include temporomandibular joint disease, Paget’s disease of the bone, thyrotoxicosis, anemia, sickle cell disease, endolymphatic sac tumors, high cardiac output, loud cardiac murmurs, labyrinthitis, perilymphatic fistulas, hydrocephalus, congenital neurosyphilis, palatal myoclonus (palatal tremor), patulous eustachian tube, middle ear myoclonus, and tensor tympani muscle spasm [52,71,103,117]. Unusual stereotyped episodes of oscillopsia and bilateral “sparking” tinnitus occurring in a cyclic (every 100 ± 10 seconds) fashion have been described (periodic saccadic oscillations and tinnitus), during which cycles of disconjugate opsoclonus, square-wave jerks, and saccadic dynamic overshoot disrupt stable fixation (see Chapter 8) [165]. It is postulated that the lesion responsible episodically disrupts saccade-related neurons and central auditory neurons in the pons.
ASSOCIATED SYMPTOMS SUGGESTING CENTRAL NEUROLOGIC DYSFUNCTION
Symptoms and signs suggesting brainstem, cerebellar, or cranial nerve dysfunction (e.g., diplopia, dysarthria, perioral numbness, drop attacks, and ataxia) localize the lesion to the central pathways. Associated auditory hallucinations suggest temporal lobe disease.
ETIOLOGIC SEARCH
An accurate history should reveal any associated viral infection, head or neck trauma, barotrauma, toxin or drug exposure, alcohol abuse, endocrine and metabolic diseases, cardiovascular disease, or previous luetic infection.
All patients should have a complete otologic and audiologic evaluation and a detailed neurologic evaluation. This examination should stress the following:
1. Blood pressure evaluation in both arms (including tests for orthostatic changes) is done with a search for cervical bruits and cardiac arrhythmias.
2. A detailed cranial nerve examination is carried out, including repetition of whispering words and numbers, tuning fork evaluation of hearing, and examination of ocular movements, including smooth pursuit, saccades, and fixation suppression, with special investigation for spontaneous or gaze-evoked nystagmus and presence or absence of skew deviation [21,27,87,169].
3. Cerebellar testing, especially evaluation of gait and station abnormalities, is performed.
4. Evaluation of vestibular control of balance and movement is done. With the Romberg test (standing with eyes closed and feet together) the patient tends to fall to the side of vestibular hypofunction. Taking a few steps with the eyes closed, the patient tends to veer to the side of vestibular involvement. This phenomenon can be highlighted by asking the patient to take three steps forward and three steps backward several times with the eyes closed. The tendency to veer to one side results in a progressive deviation to the impaired side. Instead of walking in a line forward and backward, the trajectory of the patient’s steps resembles a star (“star walking”). This abnormality may be present when all other clinical tests of vestibular function are negative. To examine the effect of vestibular function on distal movements, the patient may be asked to place the pointed index finger of the outstretched arm on top of the examiner’s finger. The patient moves his or her arm in an ample arc from above her head to meet the examiner’s finger placed in front. Consistent past-pointing is found with both hands to the side of a hypofunctional vestibular apparatus.
5. Provocative tests are designed to induce symptoms or positional nystagmus: postural changes, head-thrust test (head-impulse test), head turning, sudden turn while walking, hyperventilation, provocative positioning maneuvers (Nylén-Bárány or Dix-Hallpike test), Valsalva’s maneuver, and caloric testing.
Localization of Lesions Causing Deafness and Vertigo
Localization of Lesions Causing Sensorineural Deafness
CEREBRAL LESIONS
The human auditory cortex located in the superior temporal gyrus along the Sylvian fissure (Brodmann’s areas 41, 42, and 22) is subdivided into an auditosensory region (Brodmann’s area 41) and an auditopsychic region (Brodmann’s areas 42 and 22).
Lesions of the auditory cortex do not cause complete deafness, even when bilateral. A subtle hearing impairment may be seen with unilateral lesions, but this is more often characterized by difficulties in localizing sounds. Unilateral dominant posterior temporal lesions or bilateral temporal lesions affecting Heschl’s gyri may cause pure word deafness. Pure word deafness, also known as auditory verbal agnosia, is characterized by the inability to understand the spoken language despite normal auditory acuity. In this condition, reading, writing, naming, and comprehension of nonlanguage sounds are also preserved [59,107]. Patients with bilateral lesions of the auditory cortical regions manifest a spectrum of disorders, ranging from cortical deafness to generalized auditory agnosia, selective auditory agnosia, pure word deafness, amusia, and milder disturbances in the temporal analysis of sounds [118]. The severity of the clinical picture depends on the extent of involvement of the primary temporal processing system.
A number of patients have been reported who had severe hearing loss after bilateral temporal or temporoparietal lesions [5,62,78,162,181] or bilateral subcortical lesions [162]. In most cases, however, the severe hearing loss is eventually resolved, with only minor residual audiometric deficit accompanied by varying degrees of impairment in their ability to interpret nonverbal as well as verbal sounds (word deafness or auditory agnosia) [162]. With dichotic listening tasks, there is poorer performance on stimuli presented to the ear contralateral to a lesioned Heschl’s gyrus. Left hemispheric lesions predominantly impair speech discrimination, whereas right hemispheric lesions predominantly impair complex-pitch discrimination. Lesions in the prenatal period cause the same type of deficits but to a lesser degree than damage occurring in adulthood [132].
Irritative lesions of the temporal cortex may result in subjective auditory hallucinations. Auditory hallucinations may be simple (e.g., tinnitus) or complex (e.g., voices, music). These auditory sensations are most often referred to the contralateral ear and occur more frequently with irritative lesions of Brodmann’s areas 42 and 22 than with lesions of Brodmann’s area 41. Partial complex seizures of temporal lobe origin may start with auditory or vertiginous auras, suggesting an auditory cortical origin for the epileptiform phenomenon [72,108].
BRAINSTEM LESIONS
In general, because of the binaural representation of the ascending auditory tracts above the level of the cochlear nuclei, brainstem lesions involving the auditory pathways do not cause hearing impairment. Bilateral hearing loss may occur with severe bilateral brainstem lesions (e.g., hemorrhage or infarction) and has been described with lesions of the inferior colliculus, trapezoid bodies, pons, midbrain tegmentum, medial geniculate bodies, and cochlear nuclei. Inferior collicular lesions are rare causes of central deafness [76,157,171]. Sudden auditory illusion of paracusis (hyperacusis) and palinacusis (perseveration of sounds) have been reported with small hemorrhagic lesions of the medial geniculate body [56]. Associated brainstem findings dominate the clinical picture. Neurologic localization may be assisted by brainstem auditory-evoked potentials and magnetic resonance imaging. Sudden bilateral hearing impairment, occasionally associated with tinnitus and vertigo, was described in 7 of 503 patients with vertebrobasilar occlusive disease; 4 of these patients were in a locked-in state [77].
Pineal and midbrain tumors may also cause sudden and complete bilateral deafness (central stem deafness of Brunner [158]) presumably because the auditory pathways in this region are closely packed together in the “isthmus acousticus” [156]. Brainstem lesions in the lower midbrain or rostral pontine tegmentum may also cause auditory hallucinations associated with hearing loss and a clear sensorium, likely due to interruption of the central auditory pathways producing “release-type” hallucinations [31]. Brainstem auditory hallucinosis has also been described with lower pontine tegmentum hemorrhages [92,95].
PERIPHERAL NERVE LESIONS AND THE CEREBELLOPONTINE ANGLE SYNDROME
Peripheral cochlear nerve lesions account for partial or complete deafness, often associated with ipsilateral tinnitus. Deafness is most prominent for high-frequency tones and may be secondary to trauma (e.g., basal skull fracture), infections (e.g., syphilis, bacterial infections), drugs (e.g., streptomycin, neomycin), aneurysms of the anterior inferior cerebellar artery, or tumors of the cerebellopontine angle (e.g., vestibular schwannomas, epidermoids, meningiomas, arachnoid cysts). Nearby cranial nerves (e.g., V, VI, VII, IX, X, and XI) may be affected; their involvement assists in localizing the lesion.
The cerebellopontine angle syndrome is commonly caused by a vestibular schwannoma (acoustic neuroma) [67]. Other conditions accounting for this syndrome include meningiomas, congenital cholesteatomas, arachnoid cysts, epidermoids, lipomas, vascular loops (anterior inferior cerebellar artery, posterior inferior cerebellar artery), vertebrobasilar dolichoectasia, aneurysms, arteriovenous malformations, and vascular tumors. Commonly, but improperly called acoustic neuromas, these tumors originate from the vestibular Schwann cells of the eighth cranial nerve in the internal auditory canal at the glial-Schwann cell junction. Vestibular schwannomas account for approximately 2% to 8% of all intracranial tumors with an incidence of approximately 1:100,000. They usually present with insidious and progressive sensorineural hearing loss with early loss of speech discrimination and tinnitus. In a small percentage of cases (6%–10%) deafness may occur suddenly, most likely due to intratumoral hemorrhage or internal auditory artery occlusion. A sense of imbalance, unsteady gait, or disequilibrium is a more frequent complaint than vertigo, a disease that may be present in 20% of patients. Other common symptoms include tinnitus, headaches, and facial paresthesias [54]. As the tumor grows, the internal auditory meatus progressively widens, and complete ipsilateral nerve deafness ensues (the tinnitus subsiding as the deafness progresses). With medial tumor growth, neighboring cranial nerves are affected, and eventually brainstem and ipsilateral cerebellar compromise occur with very large tumors. Progressive tumoral enlargement may account for hydrocephalus or symptoms and signs of increased intracranial pressure.
Dysfunction of neighboring cranial nerves varies according to the direction of tumoral growth. With anterior extension, the trigeminal nerve (facial numbness, paroxysmal facial pain, depressed ipsilateral corneal reflex) and the abducens nerve (weakness of ocular abduction, horizontal diplopia) are compromised. With posteroinferior tumoral extension, cranial nerves IX and X (dysphagia, absent pharyngeal reflexes, vocal cord paralysis) and cranial nerve XI (ipsilateral sternocleidomastoid and trapezius paresis) may be involved. In either case, the facial nerve is usually involved, resulting in facial paresis, loss of taste on the ipsilateral anterior two-thirds of the tongue, decrease in ipsilateral tearing, and, rarely, hypesthesia of the posterior wall of the external auditory canal (Hitselberg sign), which is innervated by a sensory branch of the facial nerve, and hemifacial spasm [67,68,111,113,133]. In patients presenting with bilateral vestibular schwannomas, neurofibromatosis type 2 should be suspected [112,179].
Hearing loss (usually bilateral and associated with tinnitus) may occur in association with multiple branch retinal artery occlusions and encephalopathy (Susac’s syndrome) [161]. This triad of microangiopathy of the brain and retina with hearing loss occurs exclusively in young women. Sensorineural deafness and tinnitus have also been reported in cases of vertebrobasilar occlusive disease [90,100,183]. Sensorineural hearing loss may also occur in cases of bacterial meningitis, syphilis, and several viral infections including herpes zoster oticus, measles, mumps, human immunodeficiency virus (HIV), autoimmune labyrinthitis [102,115,150], and Refsum’s disease, a rare autosomal recessive condition resulting in the accumulation of phytanic acid and characterized clinically by a demyelinating neuropathy, pes cavus, cerebellar ataxia, anosmia, and sensorineural deafness [180].
Localization of Lesions Causing Vertigo
Physiologic and clinical vertigo syndromes are commonly characterized by a combination of phenomena involving perceptual, ocular motor, postural, and vegetative manifestations: vertigo, nystagmus, ataxia, and nausea [22]. Vertigo is an illusion of movement resulting from misinformation of cortico spatial orientation. Nystagmus arises from a direction-specific imbalance in the vestibulo-ocular reflex. Ataxia (or postural imbalance) results from inappropriate or abnormal activation of vestibulospinal pathways. Nausea and vomiting develop from chemical activation of the medullary vomiting centers [22].
Localizing lesions causing vertigo may be approached by considering three general categories: peripheral causes (vestibular labyrinthine disease), central causes (dysfunction of the vestibular connections), and systemic causes (e.g., endocrine, hemopoietic, metabolic diseases) [44,50,63,164].
PERIPHERAL CAUSES OF VERTIGO
Lesions of the semicircular canals induce rotatory sensations, whereas disease of the otolith system (utricle and saccule) produces linear sensations of tilt or levitation. In acute vertigo due to labyrinthine disease, the diseased side may be the more active of the two (irritative phase) for some hours or even days, but it soon becomes less active (paretic phase). When the eyes are closed, patients feel a rotational sensation toward the side opposite to the paretic labyrinth. By contrast, in the paretic phase the eyes tend to deviate slowly toward the side of the lesion, and to that side, patients tend to past-point and fall when standing with eyes closed. Patients with severe vertigo feel most comfortable lying on one side, usually with the affected ear uppermost, perhaps to use otolith inputs in order to decrease the imbalance between the semicircular canals. In patients with labyrinthine disease, acoustic stimuli may induce paroxysms of vertigo, oscillopsia, postural imbalance, the ocular tilt reaction, and nystagmus (Tullio’s phenomenon), perhaps through utricular stimulation [43].
Peripheral vestibular syndromes are usually of short duration and characterized by severe, often paroxysmal vertigo accompanied by auditory dysfunction (tinnitus and hearing loss). Nystagmus is often present and is characteristically unidirectional (fast phase “away from” the side of the lesion), horizontal rotatory (never vertical or exclusively rotatory), and inhibited by visual fixation. The subjective environmental twirl, past-pointing, deviation of the outstretched hands, and fall associated with the Romberg’s maneuver are toward the slow phase of the nystagmus (toward the side of the lesion). The peripheral vestibular syndrome is therefore complete (has all of the clinical elements of vestibular dysfunction, e.g., vertigo, nystagmus, deviation of the outstretched hands, Romberg’s sign, and so on) and congruent (all the “slow deviations” are toward the same side, i.e., ipsilateral to the responsible lesion).
Unilateral total loss of horizontal semicircular canal function (i.e., canal paresis) may be detected by having the patient fixate on a stationary target while the examiner turns the head from side to side [65]. In normal individuals, no saccades (quick eye movements) are noted, indicating that the subject’s gaze remained fixed on target. In patients with total unilateral canal paresis, one large or several small oppositely directed, compensatory, refixation saccades occur when the head is rotated toward the lesioned side [65].
Acquired vestibular areflexia, especially when bilateral, may also result in head movement– dependent oscillopsia, which is an illusory movement of the visual world that occurs only during head movement [167].
Benign Paroxysmal Positioning Vertigo
Positional vertigo of the benign paroxysmal type [8], also known as benign paroxysmal positional vertigo, or more appropriately benign paroxysmal positioning vertigo (BPPV), is a very common mechanical disorder of the inner ear in which brief attacks of acute and severe vertigo with concomitant nystagmus and autonomic symptoms is precipitated by certain head movements (often while patients turn in bed). Cochlear or other neurologic symptoms are typically absent, and the symptoms usually abate after 3 to 6 months. Nearly all patients have at least one exacerbation after an initial remission [8]. Patients are otherwise asymptomatic between bouts [20]. BPPV can affect one or more semicircular canals, although involvement of the superior semicircular canal is extremely rare. Commonly, BPPV involves the posterior semicircular canal. BPPV may follow head trauma, viral labyrinthitis, Ménière’s disease, migraines, or inner ear surgery, but most cases (50%–70%) are primary or idiopathic and best explained by the canalithiasis or cupulolithiasis theory [153]. Most cases of posterior canal BPPV are due to canalithiasis [4,139]. Stray otoconial (calcium carbonate crystals) particles detached from the otoconial layer (by degeneration or trauma) gravitate and settle on the cupula of the posterior semicircular canal (PC-BPPV), causing it to become heavier than the surrounding endolymph and thus sensitive to changes in the direction of gravity [20,153]. After rapid head tilt toward the affected ear or after head extension, when the posterior semicircular canal is moved in the specific plane of stimulation, an ampullofugal deflection of the cupula occurs, with the development of a rotational vertigo after a short latent interval and most commonly concomitant upbeat geotropic (“toward earth”) nystagmus with the fast phase beating toward the undermost ear. The nystagmus typically adapts and fatigues after repeated positional testing [20].
Other patients (10%–30%) display the lateral or horizontal semicircular canal BPPV variant (HC-BPPV) in which there is a strong linear horizontal nystagmus beating toward the lowermost ear induced by rapid turning of the head from side to side around the longitudinal axis. The nystagmus exhibits short latency without fatigability, and often reverses its direction on the pathologic side. The vertigo can be induced by turning the head to either side in the supine position, and is always more prominent on the pathologic side. The horizontal variant of BPPV tends to resolve more quickly than the posterior canal BPPV. Two variants of HC-BPPV have been described: canalithiasis (floating otoconial debris) and cupulolithiasis (fixed otoconial debris) of the HC [172]. Most cases of HC-BPPV are due to cupulolithiasis [79]. Some patients exhibit a combination of PC-BPPV and HC-BPPV [10,116,138,159].
Other causes of positional vertigo include trauma, infection, ischemia [61], demyelinating disease, neurosarcoidosis [173], Chiari malformations, posterior fossa tumors, decompression sickness [38], maxillary dental implants [140], use of whole-body vibration training plate [2], intense physical activity [57] including mountain biking [170], cochlear implantation [104], and perilymphatic fistulas. Perilymphatic fistulas may be congenital or acquired. Perilymphatic fistulas usually follow barotrauma resulting in an abnormal communication between the perilymphatic space and the middle ear. Barotrauma can occur during driving, playing, or following violent bouts of coughing or sneezing. Patients experience attacks of imbalance or vertigo with increase in pressure in the ear.
The provocative positioning maneuver (Dix-Hallpike or Nylén-Bárány maneuvers) (patient is briskly moved from the seated position to a position where the head is hanging 45 degrees below the horizontal and rotated 45 degrees to one side) (Fig. 11.2) allows for a differentiation between a peripheral or a central origin for positional vertigo.
In normal individuals, these maneuvers do not induce nystagmus. With peripheral lesions, vertigo, nausea, vomiting, and nystagmus appear several seconds (1–15 seconds) after the head position is changed (latency of response due to the period of time for the otoconial mass to be displaced). The nystagmus is usually torsional, with the upper pole of the eye beating toward the ground (geotropic). Fatigue with repeated positioning is seen. The nystagmus fatigues and abates within 10 seconds of appearance (fatigability due to dispersion of particles in the endolymph), and when the patient is rapidly brought back to a sitting position, the nystagmus beats maximally in the opposite direction (rebound). With repetition of the maneuver, the nystagmus becomes progressively less severe (habituation).
A central lesion should be suspected and further investigations initiated when (a) the positioning testing maneuver is positive with the head turned to either side, (b) an ageotropic positional nystagmus does not change to geotropic, (c) the nystagmus changes direction immediately after the shift in position and remains for as long as the head is down, (d) the nystagmus is unaccompanied by nausea or vomiting, and, if present, vertigo is mild and lasts <60 seconds, and (d) the nystagmus does not display features of adaptability or fatigability [16].
Matutinal vertigo (vertigo precipitated by the act of getting to one’s feet on awakening in the morning or sometimes on turning over preparatory to rising) may be central or peripheral and, therefore, of no localizing nature [14]. Matutinal vertigo is most frequently seen with disorders in which positional features are prominent, and it is often prevented by having patients sleep in a semi-upright position and patients being cautious about easing out of bed in the morning [14].
Peripheral Vestibulopathy
This term refers to conditions characterized by acute or recurrent attacks of episodic vertigo caused by extramedullary disorders of the vestibular system [44]. Precise knowledge of the site or nature of the lesion is unknown. These conditions encompass acute vestibular neuronitis, acute labyrinthitis, epidemic vertigo, and viral labyrinthitis.
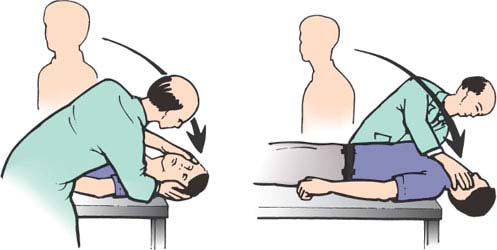
FIG. 11.2. The Dix-Hallpike or Nylén-Bárány maneuver.









