Cranial Vascular Anatomy
Neuroendovascular techniques not only have become an accepted modality for the treatment of neurovascular disease but also continue to evolve at an accelerated pace with progressive advancements in technology and material science. However, neuroendovascular therapies could never have developed without knowledge of the fundamentals, namely neurovascular anatomy. Thus it is appropriate that the first two chapters of this book be designed to familiarize you with basic concepts of vascular embryology and ultimately cranial and spinal neurovascular anatomy.
As with most specialties, jargon and vernacular are important to establish at the beginning so that the reader understands the concepts. Neurovascular anatomy is no different, and the language of description must first be defined so the reader may develop an internal understanding of the text that follows.
Although the neurovascular system has specific components that make it unique, it has the same fundamental building blocks as the other vascular systems in the body. More specifically, the neurovascular network is made up of arteries, capillaries, and veins, all with various sizes and functions. Each vessel has both functional and physical properties that make it unique.
The vascular tree is a continuous network made up of a multitude of varying-sized vessels (Table 1.1) that are continuous with one another. Arteries characteristically share the features of a well-defined lumen maintained by a muscular vessel wall, while veins are more commonly thin-walled but with large amounts of elastin fibers without large amounts of smooth muscle cells.1 Large arteries have disproportionately thin walls for the size of their lumen, great elasticity, a thick tunica media (Table 1.2), and an adventitia with a high concentration of collagen and elastin. Medium-sized arteries, which constitute the majority of the named arteries of the body, have a greater proportion of smooth muscle fibers to elastic fibers in the tunica media, a thinner tunica intima than large arteries, and a prominent internal elastic membrane with a basement membrane. The adventitia of medium-sized arteries is relatively thick, with a high concentration of collagen fibers. Arterioles, which are much smaller in diameter, have a thin tunica media composed of an incomplete layer of smooth muscle. The adventitia is also thinner than that of large arteries, and the external elastic membrane is poorly defined or even absent. Capillaries, on the other hand, have very thin walls composed of an endothelium and delicate basement membrane and are classified as either fenestrated or non-fenestrated (Table 1.3). Blood flow through capillaries is slow, which allows diffusion to occur bi-directionally with the surrounding interstitial fluid
After an exchange of nutrients and wastes through the capillary walls, the blood flow continues through the veins (Table 1.1). The main function of the veins is to receive blood from tissues via the capillary system and return it to the lungs, where the blood can once again become oxygenated, and to the other filtration systems in the body, namely the liver. A description of the exchange processes is beyond the scope of this text. Veins are generally smaller in outer diameter than the corresponding arteries but generally have larger inner diameters and can accommodate a much larger volume of blood.
Table 1.1 The Neurovasculature Is Classified According to Size
Vessel | Size | Function |
Large or elastic arteries | 0.4 cm – 2.5 cm | Move volumes of blood throughout the body. |
Medium or muscular arteries | 40 mm – 0.5 mm | Distribute blood to body and organs. |
Small arteries or arterioles | 0.008 mm – 0.5 mm | Deliver blood at the tissue level. |
Capillaries | <0.008 mm | Exchange between blood and interstitial fluid. |
Venules | ∼0.030 mm, variable | Smallest veins, thin-walled with notunica media. Collect blood from the capillarybeds. |
Medium-sized veins | 0.030 mm – 5 mm | Thin tunica media with few smooth muscle fibers. One-way valves allowing blood to travel only in one direction, toward the heart. Peripheral skeletal muscle supplies the force to squeeze peripheral veins and push blood toward the heart. External elastic layer is composed of elastic and collagen fibers. |
Large veins | >5 mm | Thick layer of elastic and collagenous fibers forming the tunica externa. Thin tunica media; intrathoracic pressure delivers blood to the heart. |
Composition | Function |
Few smooth muscle fibers High density of elastic tissue | Imparts resilience to large arteries to tolerate pressure changes with cardiac contraction. |
Fenestrated | Allow select sized molecules to diffuse through the fenestrations. |
Non-fenestrated | Allow solutes to pass through the vessel wall by passive and active diffusion. |
Embryology: Aortic Arch and Head and Neck Vasculature
The development of the adult vasculature requires an infinite number of steps in both the embryonic and postnatal phases.2,3 Variants in anatomical structure are common and typically a result of either the failure of regression of some embryonic components or overly aggressive formation of others. Rarely will the knowledge of the intimate details of embryologic development of the vasculature be necessary, so the descriptions below serve only to give one the basics of how these processes are initiated and the basic results. Any further detail should be gleaned from texts written specifically on this topic.
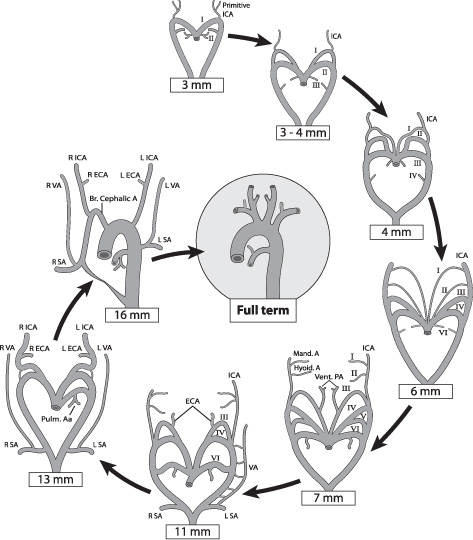
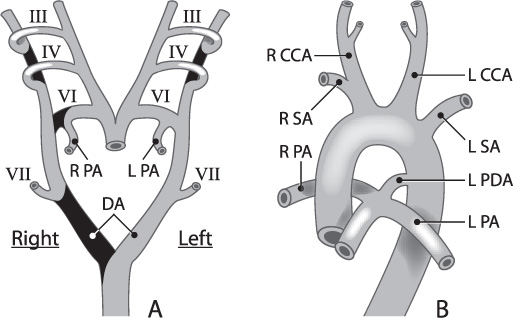
Vasculogenesis begins day 18 post conception in the splanchnic mesoderm of the embryonic disc, forming small capillaries. Small capillaries then coalesce into larger cords running throughout the fetus. During the fourth week of gestation, the great vessels begin to develop. The aorta develops as a series of aortic arches, as in the jawless fish that gave rise to land-based higher mammals (Fig. 1.1). Arches develop as a series of evolutions and regressions that result in the development of the great vessels and the vessels to the head and neck. There are five pairs of pharyngeal arches: 1, 2, 3, 4, and 6; the fifth arch either never develops or regresses quickly, so it does not contribute to the development of the vascular tree (Fig. 1.2A). These pairs of aortic arches fuse below as a single dorsal vessel.
Aortic Arch and Extracranial Circulation
The development of the aortic arch (Table 1.4) in the embryonic stages can be divided into two distinct stages, the branchial and the postbranchial.3,4 The branchial stage, which is often seen to cease in lower vertebrates, involves a series of overlapping steps in which the six pairs of aortic arches, with the pharyngeal arches, begin to form the more-defined cephalic vessels (Fig. 1.2B). Anomalies at this stage include the failure of regression of the primitive maxillary or hypoglossal arteries. The postbranchial stage involves the evolution of the adult arterial pattern (Table 1.5) from branchial remnants, which persist after successive interruption by the pharyngeal structures and subsequent forming and regressing of the remaining aortic arches. The most common anomaly during these stages is a failure of the regression of the primitive trigeminal artery. For a more detailed timeline of the steps of embryonic arterial vasculature development, please refer to Table 1.6.
Fig. 1.2 Progressive development of the cephalic arteries (carotid, subclavian, and pulmonary arteries) from the aortic and pharyngeal arches (III to VII) is illustrated here from fetal to final adult form.
Table 1.4 Normal Development of the Aortic Arch and Great Vessels
Embryonic origin | Primitive artery | Final artery |
Bulbus cordis | Truncus arteriosus |
|
Aortic sac | Ventral aorta |
|
Dorsal aortae | Carotid arteries (distal segments) |
|
Cervical plexuses | Cervical intersegmental arteries |
|
Dorsal plexuses | Longitudinal neural arteries |
|
Aortic arches |
|
|
I | Maxillary arteries | Portions of distal ECA (maxillofacial division, mandibular and infraorbital branches). |
II | Stapedial arteries | Stapedial arteries. |
III | Carotid arteries (proximal) | CCA: proximal ICA and ECA. |
IV | Right and left primitive aortic arches | Right: brachiocephalic and proximal SCA. Left: aortic arch, proximal descending aorta, left subclavian artery. |
VI | Ductus arteriosus | Left and right pulmonary arteries.* |
Abbreviations: CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; SCA, superior cerebral artery.
Variations and Anomalies
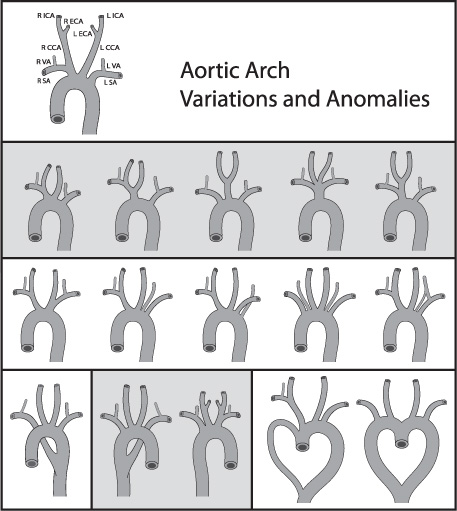
As many in neuroendovascular surgery will attest, there is a great variety in the anatomical construction of the aortic arch, and overlooking this fact can provide numerous challenges during catheterization. As such, some variants of the aortic arch need to be known by the neuroendovascular surgeon (Table 1.7). The most common aortic arch configurations are the following: (1) the “bovine” arch, where there are essential two emanating trunks from the superior surface of the arch, one consisting of a common origin of the left common carotid artery and the brachiocephalic trunk and the other being the left subclavian artery; (2) the origin of the left vertebral artery directly from the aortic arch; and (3) the origin of the right subclavian artery off the ascending aorta (Fig. 1.3).5
Segments | Description | Branches |
Ascending | Begins at the semilunar valve of the left ventricle and extends to the transverse curve. | Right and left coronary arteries originate from left anterior and posterior sinuses just above the aortic valve. |
Transverse | Extends from the transverse curve to the left subclavian artery. | Brachiocephalic trunk Left CCA Left SCA |
Isthmus | Fetal aorta narrows distal to L SCA and continues to the descending aorta. |
|
Descending | Starts left of the fourth thoracic vertebra and descends toward diaphragm. |
|
Abbreviations: CCA, common carotid artery; SCA, superior cerebral artery.
Fig. 1.3 Aortic arch variations. Depicted here are the numerous aortic arch variations and anomalies. CCA, common carotid artery; SA, subclavian artery; ICA, internal carotid artery; VA, vertebral artery.
Table 1.6 Timeline of Arterial Development
Time | Event |
Day 22 | Maxillomandibular arches (first pharyngeal arch) develop. |
Day 26 | Second aortic arch appears as first pair regresses. |
Day 28 | Third and fourth arches appear. |
Day 29 | Sixth arch appears. Second arch begins to regress. Stapedial artery develops from proximalend of second arch and contributes to the ECA. |
Days 21-28 Day 35 | Primitive carotid arteries deliver intracranial blood flow. They arise from the ventral aorta and third arch. Distally, the primitive carotid artery distal segment divides into cranial (future ACA) and caudal (future PCOM) segments. Dorsal segments connecting the third and fourth aortic arches regress, so that the entire blood supply of the third arch is delivered to the head. |
Week 5 | Third, fourth, and sixth arches are well developed, and the ICA is supplied entirely by the ventral aorta and third arch. The vascular plexuses dorsal to the third and fourth arches fuse to become the basilar artery. Transient anastomoses between the ICA and CCA and plexiform longitudinal neural arteries are trigeminal, otic, hypoglossal, and proatlantal arteries. ECA origin is unclear, but likely related to the first and second arch remnant and the ventral end of the third arch. |
Week 6 | Regression of longitudinal neural-ICA anastomoses. The seven cervical intersegmental arteries coalesce to form the VA. The first six proximal connections and dorsal aorta regress. The seventh segment becomes the subclavian artery. The pulmonary artery initially sprouts from the fourth arch and reconnects to the sixth arch. |
Week 7 | The left fourth aortic arch and dorsal aorta result in the definitive aortic arch and cranial segments of the descending aorta. The brachiocephalic artery is a remnant of the third and fourth arches. BA finishes coalescing and anastomoses interiorly with the VA. |
Week 8 | Near-fnal configuration of aortic arch and great vessels. Ductus arteriosus remains patent, providing communication between the pulmonary trunks to the proximal descending aorta. |
Abbreviations: ACA, anterior cerebral artery; ECA, external carotid artery; ICA, internal carotid artery; PCOM, posterior communicating artery; VA, vertebral artery.
Shared brachiocephalic trunk/Left CCA origin | Most common: the “bovine” configuration seen in an estimated 27% of patients. For 7% of patients, the left CCA originates from the proximal brachiocephalic trunk. |
L SCA/CCA from a common origin and form a left brachiocephalic trunk | Found on 1% to 2% of patients who have angiograms. |
Right aortic arch | Aberrant left SCA producing mirror image branching. Associated with congenital cyanotic heart disease. |
Double aortic arch | Secondary to incomplete regression of paired fetal structures. |
Aortic spindle | A narrow segment from the left SCA and descending aorta persists into adulthood. This junction between the isthmus and spindle is marked by an inferior indentation. |
Ductus diverticulum | Seen in 9% of adults. Incomplete closure of the ductus arteriosus. Identified as a bulge at the anteromedial aspect of aortic isthmus. |
Abbreviations: CCA, common carotid artery; SCA, superior cerebral artery.
Intracranial Circulation
The cerebral vasculature is formed when angioblasts coalesce in areas around the cranium and form plexuses. These plexuses ultimately connect with one another, forming a single plexus covering the entire neural tube. By a series of resorptions of some components of the plexus and expansions of other components, the adult arterial configuration is developed. The internal carotid artery ascends from the aortic arches in the neck and joins the cerebral plexus. The carotid also develops branches of its own, the most important of which include the primitive trigeminal artery to supply the hindbrain through the longitudinal artery, as well as a bud that will become the ophthalmic artery in the adult. By day 30, a second communication between the internal carotid artery (ICA) and the rostral longitudinal artery develops that will later become the posterior communicating artery (PCOM). By day 33 the longitudinal arteries have joined, forming the basilar artery, and the vertebral arteries have formed by the anastomoses of the cervical segmental arteries. By day 35 the anterior cerebral arteries, middle cerebral arteries, and internal carotid artery branches are forming. By day 44 the intracranial arteries are in the final position, and a complete circle of Willis has formed. By 70 days the arterial system is in a mature form, with arterial size and caliber still fluctuating, but with little change in organization.3
Circle of Willis Development
The circle of Willis develops to provide a collateral circuit to connect the anterior and posterior, and the right and left intracranial circulations (Table 1.8). When a complete circle develops (described later), a single patent carotid artery or vertebral artery can maintain the brain’s blood supply.
Venous Development
The development of the venous system is the more complex embryological tale and has been noted throughout history to involve significantly more numerous and complex stages.2,5 The development of the venous system lags behind arterial evolution in all embryonic stages and continues into the postnatal period. Angiographic evidence of embryonic venous structures can be seen in those who are imaged in the infant stage. As such, vascular malformations present at birth may be the result of failed regression of embryonic structures, and the venous pattern observed might not be one the physician recognizes.
The precursors of the venous system begin to take form in the third and fourth weeks. The intracranial venous system begins with a capillary plexus as well as an anterior, middle, and posterior dural plexus draining the neural tube into a lateral sinus known as the head sinus. This sinus drains into the anterior cardinal vein, a member of the primitive venous system. By day 40 post-gestation, a longitudinal sinus connects the anterior, middle, and posterior sinuses and drains into the internal jugular vein. A section of the anterior venous plexus will eventually become the superior sagittal sinus. The anterior and middle plexuses will join to form the transverse sinus, and the middle and posterior will join to form the sigmoid sinus. Over time this plexus will coalesce into the superior sagittal and transverse sinuses. The venous system lags behind arterial development considerably, and it continues to develop throughout gestation and even after birth.
The primitive extracranial venous system is composed of the cardinal veins, in which the anterior division is responsible for the drainage of the head and neck, while the posterior division drains the body. In the areas where the anterior and posterior cardinal veins converge, common cardinal veins are seen, which are the precursors of the internal jugular veins. The subclavian veins develop as bilateral vein buds in the upper extremities and ultimately anastomose with the anterior cardinal veins to form the brachiocephalic veins.
Table 1.8 Circle of Willis Developmental Segments
Cranial division | Initiates as primitive olfactory artery and gives rise to definitive ACA and MCA. ACOM forms from a plexiform vascular network. |
Caudal division | Anastomoses with dorsal longitudinal neural arteries and regresses to form the PCOM. Supplies precommunicating segment of PCA BA from pair of dorsal longitudinal arteries. |
Abbreviations: ACA, anterior cerebral artery; ACOM, anterior communicating artery; MCA, middle carotid artery; PCOM, posterior communicating artery.
The intracranial venous drainage develops from a series of plexiform dural channels that will ultimately form several important venous structures in the adult. The superior sagittal sinus is formed from a plexiform channel of veins covering the telencephalon and diencephalon. As the telencephalon grows posteriorly, the venous channels are stretched in a sagittal plane leading to the cortical draining veins observed in the adult. The cavernous sinus and its associated venous structures are derived from embryonic structures independent of intracranial drainage. The formation of the deep venous circulation occurs late in the embryonic stages. For a more in-depth discussion of the exact steps of embryonic venous vasculature development, please refer to Table 1.9.
Adult Arterial Vasculature: Extracranial Circulation
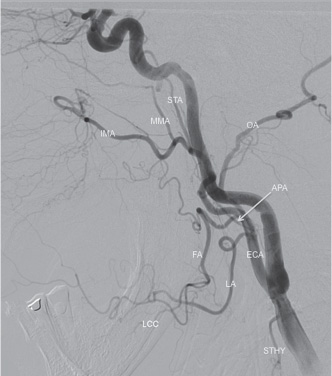
The extracranial circulation normally consists of a series of arteries that pass through the neck on the way to the cranium or arteries that supply extracranial structures in the head and neck.3,4,6 The extracranial circulation consists of the bilateral common carotid arteries, cervical carotid arteries, external carotid arteries, and the vertebral arteries. The carotid arteries begin as a common trunk called the common carotid artery. They then split into the internal carotid artery that supplies the intracranial structures and the external carotid artery (ECA) that supplies the head and neck (Fig. 1.4). The left common carotid artery originates directly from the aortic arch, while the right common carotid artery is a branch of the brachiocephalic trunk behind the right sternoclavicular junction. The common carotid ascends in the neck for some distance before dividing. The most common location of the carotid bifurcation is at the C3–4 vertebral body level; however, it can occur from C2 to T3. It is always important to know where the carotid bifurcation is located, because much pathology can be localized to this area, for example, atherosclerotic disease leading to carotid stenoses. Endovascular surgeons may also encounter a non-bifurcating carotid artery, in which the ECA originates directly off the aorta as a carotid trunk. The vertebral and internal carotid arteries do not typically have any branches outside of the skull, while the external carotid supplies structures in superficial and deep head and neck.
Adult vein | Embryonic vein |
| Anterior cardinal veins drain the head and neck in the third and fourth weeks. |
| Posterior cardinal veins drain the body. |
Internal jugular vein | Common cardinal veins formed by the anterior and posterior cardinal veins. |
External jugular vein | Formed from a capillary plexus. |
Brachiocephalic vein | Subclavian veins develop as bilateral vein buds in the upper extremities and anastomose with the anterior cardinal veins to form the brachiocephalic vein. |
Superior vena cava | Formed by the left and right brachiocephalic veins. |
The internal carotid artery runs in the carotid sheath after bifurcating from the common carotid. In the carotid sheath, the internal carotid artery lies anteromedially to the internal jugular vein, carrying with it cranial nerves IX, X, XI, XII and postganglionic sympathetic fibers. The cervical carotid artery is congenitally absent in 0.1% of the population; most of the cases are unilateral, but some rare cases can be bilateral. The cervical internal carotid artery can be injured from anterior and lateral neck tumors, infections, or trauma, resulting in pseudoaneurysms, dissections, and fistulae. Atherosclerotic disease is the most common disease of these vessels, most often found in the aged population and treatable with carotid endarterectomy or endovascular stent placement. All of these acute and chronic vessel diseases place the patient at risk of ischemic or thromboembolic stroke in the anterior cerebral circulation, making early diagnosis and treatment critical.
At the carotid bifurcation, the external carotid artery is initially located anteromedially to ICA and then courses in a posterolateral direction. The internal jugular vein (IJV) runs posterior and lateral to these structures on its descent to meet the subclavian. The ECA is covered superficially by the sternocleidomastoid muscle and is crossed by the hypoglossal nerve in the upper cervical region. The ECA is responsible for the blood supply to the majority of the face, scalp, external and skull base auditory structures and the dura. The ECA divides into several terminal branches emanating from its main trunk, coursing both superficially, as well as deeply (Fig. 1.5). These arteries supply many important structures in the head and neck, as well as form collaterals with intracranial circulation (Table 1.10).
The vertebral arteries are paired vessels originating off the brachiocephalic trunk on the right and the left subclavian artery. In rare cases the vertebral artery may originate from the aortic arch, common carotid artery, or internal carotid artery. After their origin, the vertebral arteries normally enter the bilateral spinal foramina transversaria at C6. The artery remains contained within the foramina until the C2 body and is surrounded by a complex venous plexus. At the level of C2, the paired vertebral arteries leave the foramina and course laterally and posteriorly, then ascend cephalad, through the foramen transversarium of C1. The artery then courses posteromedially over the arch of C1, before turning anteromedially to pierce the atlanto-occipital membrane, becoming intracranial. Often the vertebral arteries are unequal in caliber. Fifty percent of patients are left-side dominant (having a larger left vertebral artery), twenty-five percent are right-side dominant, and twenty-five percent are co-dominant. The cervical vertebral arteries are divided into four segments (Table 1.11 and Fig. 1.6).
Cervical vertebral artery injury occurs most commonly via catheter manipulations, head and neck trauma and tumors, and surgical spine fusion. As with the carotid arteries, acute injury can result in pseudoaneurysms and dissections, but atherosclerotic disease is also common and results in posterior circulation ischemic strokes and symptoms of vertebrobasilar insufficiency. Surgical treatment of vertebral artery disease is not very common now that endovascular options are available. The vertebral arteries are more difficult to access surgically than the carotid arteries because of the bony canal of the vertebrae (foramen transversarium) in which they travel along their course to the brain.
Extracranial Venous Drainage
Drainage of the head and neck occurs through the jugular venous system. The extracranial head and neck are drained primarily through the external jugular vein. The external jugular vein (EJV) begins high up in the neck, with the coalescence of the posterior auricular vein and the retromandibular vein. The occipital, posterior external, transverse cervical, transverse scapular, and anterior jugular veins later join the EJV to drain the face and head. Not all drainage of the face and scalp is superficial. Some veins drain deeply into the internal jugular vein rather than the external, and these include the occipital, facial, lingual, pharyngeal, and superior and middle thyroid veins (Fig. 1.7). The external jugular vein itself also communicates with the internal jugular vein through a plexus in and around the parotid gland. The external and internal jugular veins themselves communicate with a connection through the parotid gland. The EJV ultimately crosses the sternocleidomastoid muscle superficially before piercing the deep fascia to join with the subclavian vein.
Table 1.10 External Carotid Artery Branches
Superior thyroid artery (STA) | First branch of the ECA; anteroinferior, coursestoward thyroid gland. Supplies the larynx and upper thyroid. 20% arise above bifurcation, 10% from CC, 2% fromlingual artery. |
Ascending pharyngeal artery (APA) | Smallest and first posterior branch. Originates within 2 cm of the ICA/ECA bifurcation. Courses behind ICA, anterior and medial to IJV. Supplies the nasopharynx, oropharynx, middle ear, cranial nerves IX, X, XI, and meninges. Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|