Chapter 154 Disc Replacement Technologies in the Cervical and Lumbar Spine
Background Considerations
The intervertebral disc is composed of a central nucleus pulposus (containing predominantly Type 1 collagen fibrils) and a peripheral annulus fibrosis (which can consist of up to 25 lamellae of mostly Type 1 collagen) that provides support, allows some movement, resists excessive movement, and, according to some absorbs shock.1 The ability to resist axial stresses is considerable but decreases with age.2 With normal loading, the normal nucleus pulposus is transposed by vertebral body pressure to tighten the annulus and produce ligamentous intervertebral stability. With heavy loading, the rigid, spherical normal nucleus pulposus gains stable seating in the nuclear recesses, where it acts as a piston to depress the cribriform plates, bend the trabeculae, and produce the necessary stability. With return of normal loading, the vertebral body rebounds, the nucleus pulposus resumes its normal locus, and the annulus tightens. Therefore it has been recognized that the vertebral bodies are very significant contributors to the shock-absorption function of the spinal column.3
It is fairly well accepted that the degenerative changes that result from aging of the vertebral body, the annulus fibrosis, and the nucleus pulposus are likely to begin with infarction of the cribriform cartilage endplates and subsequent nutritional failure of the nucleus pulposus.4 Calcification of the endplates occurs in adulthood, and the nutrient uptake and waste elimination within the disc then becomes dependent on diffusion. This leads to anaerobic metabolism’s taking a more prominent role, leading to lactate production and an acidic environment. The proteinases become more active, resulting in further disc degeneration.5 Indeed, it has been shown that when heavy loads are applied to the intervertebral disc, the normal disc biology can be disrupted, leading to an increase in catabolic enzymes and an acceleration of intervertebral disc degeneration.6 Studies have noted increased rates of degenerative disc disease in siblings of affected persons and strong correlation in twins.
The pathophysiology of the degenerative disease process has been described by Kirkaldy-Willis.7 The progressive disease is divided into three stages based on the amount of damage or degeneration to the disc and facet joints at a given point in time. However, the cascade of individual motion segment degeneration is best thought of as a continuous process rather than as three clearly definable and separate stages.
Stage 2: Instability
The second stage is described as the instability stage. It represents more significant tissue damage and tends to occur later in life, typically between 30 and 50 years of age. Multiple annular tears and delamination of the annulus fibrosis accelerates the intervertebral disc degeneration to a point where vertebral segment instability occurs. This results in a further decline in the nuclear proteoglycan composition, with a further resulting deterioration in the water content. Collapse of the nucleus pulposus leads to increased force transfer to the annulus fibrosis. The clinical episodes now tend to be periods of back pain that are usually more intense, often last longer, and tend to require more aggressive intervention. The MRI reveals loss of intervertebral disc height, a darker disc on T2-weighted imaging, and sometimes a disc herniation.
Stage 3: Stabilization
However, MRI is positive in asymptomatic patients at least 40% of the time.8–14 About 30% of adults without low back pain have evidence of protruded disc on MRI; more than half have bulging or degenerative discs and a fifth have annular fissures.15 Therefore, the relationship among disc degeneration, the MRI appearance, and spine-related pain remains controversial, and the decision to undertake surgical management of a degenerative spinal condition is a large one and is very much patient specific. The presurgical workup must include a thorough history relating to any spinal complaints including neurologic compromise, a diligent physical examination that leads to a working diagnosis, and the appropriate subsequent confirmatory radiologic imaging. Although simple radiography is an inexpensive and readily accessible starting point, its utility is severely limited by its inability to visualize neural structures directly or indirectly, and therefore the presence or absence of neural compression is indeterminable.16,17 Nonetheless, all our potential arthroplasty patients undergo plain flexion-extension radiographs of the appropriate spinal segment to look for instability of the potential operative or another segment and to confirm that there is still movement of the proposed operative segment. Also, if there is going to be any delay in obtaining true confirmatory imaging, routine spinal radiography should be undertaken to exclude other disease processes, such as malignancy, infection, or arthritis. Interestingly, when Friedenberg and Miller compared 92 asymptomatic patients with those complaining of neck and arm pain, no difference was found between the two groups in radiographic findings, with the exception of a greater incidence of disc degeneration at C5–6 and at C6–7 in the symptomatic group.18
None of our groups have been provided with the resources to undertake psychological profiling of prospective patients.19 Nonetheless, we try to avoid operating on patients with outstanding litigation claims.
General Considerations
The elimination of motion at the functional spinal unit (two vertebral bodies, the intervertebral disc, and associated facet joints) has been the mainstay of treatment since the 1960s.26 Anterior cervical discectomy and fusion is the standard of care for relief of pain and stabilization associated with radiculopathy and myelopathy,27 with excellent long-term results. However, lest we forget, very satisfactory results have been reported with the placement of nothing at all in the disc space after anterior cervical discectomy.28,29 It was the significant concerns raised by Hillibrand30,31 and others who noted, in various series of patients who had undergone anterior cervical arthrodesis, that between 25% and 89% who were followed for a lengthy period developed new degenerative changes at adjacent levels.
The debate is complex because much of the biomechanical and clinical evidence about the cause of adjacent segment disease is anecdotal and inconsistent.32 Although intradiscal pressure and motion alterations have been found at the adjacent levels following a single-level anterior lumbar interbody fusion (ALIF) in a calf model,33 it is recognized that longer fusions, both in the lumbar spine and in the cervical spine, have not been associated with higher rates of adjacent segment disease.32,34
Indeed, the recent enthusiasm for the application of cervical plates to aid fusion might actually be related to the increase in adjacent segment degeneration. Anterior plate impingement upon an adjacent disc is likely to accelerate adjacent level changes.35 An association between adjacent level ossification and the plate-to-disc distance has been established in a retrospective review of 118 patients undergoing anterior cervical discectomy and fusion.34 We should not forget that a patient who has already developed cervical spondylosis at the most common level (C5–6) to such a degree that surgery is warranted may be predisposed to develop degeneration at an adjacent level (C6–7 or C4–5) because of the natural history of the spondylosis and independent of whether or not a fusion is performed at the original level.
Moreover, despite the frequent reference to Hillibrand’s paper30 by those promoting arthroplasty, the authors later indicated that the paper suggested that the development of adjacent segment disease may be related to the natural history of cervical spondylosis.31 Similar findings have been documented in the lumbar spine by Ghiselli and colleagues,33 who found no correlation between the length of fusion and the rate of reoperation in 215 patients following posterior lumbar fusion. They noted that segments that were adjacent to a single-level fusion had a three times higher risk for developing disease than did those adjacent to a multiple-level fusion.
Operative Procedure
ProDisc-C Artificial Cervical Disc Placement
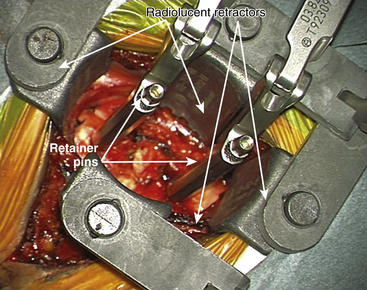
For placement of the implant it is essential to have radiolucent retractors because the procedure require visualization of most steps on x-ray.
The ProDisc-C artificial cervical disc system comes with vertebral body retainer screws. The retainer screws are inserted under x-ray guidance (lateral projection) in the upper one third of the superior vertebra and inferior one third of the inferior vertebra parallel to the respective end plates (Fig. 154-1). The screws are bicortical. The length of the screws can be calculated on the picture archiving and communication system (PACS) using a CT scan image. The retainer handle is mounted onto the screws. It is important to leave enough space between the retainer pins to allow for the height of the keel on the ProDisc-C prosthesis. It is important not to use the retainer pins to distract the disc space. Their purpose is to stabilize the segment while the various steps of preparing the bed for the implant, keel cutting, and insertion of the implant are carried out.
The primary objective of the operation is the decompression of the spinal canal and/or foramina. The annulus is incised and removed with a pituitary rongeur or curette. The disc can be removed with curettes or a high speed drill (Fig. 154-2). Good lateral exposure is necessary for the implant, and the dissection is continued laterally until the upslope of the uncovertebral joints on either side is identified. Further lateral dissection or drilling can damage the vertebral arteries. The uncovertebral joints must not be drilled to keep the segment stable after the insertion of the ProDisc-C implant.
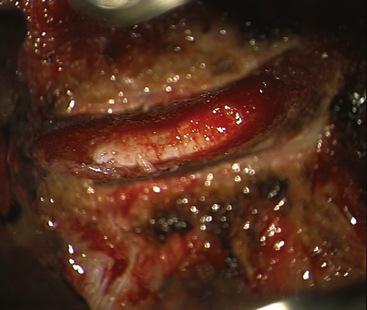
FIGURE 154-2 The posterior longitudinal ligament is excised and posterior osteophytes are removed.
(Courtesy of Mr. A. Saxena.)
Connect the trial handle to the trial implant. Ensure that the stop is fully screwed, closest to the footprint. Align the trail implant on the midline and advance the trial implant under image intensifier into the disc space.
Milling for Keel Cut Preparation
Choose the milling guide according to the height of the trial implant (Fig. 154-3). Slide the milling guide over the shaft of the trial implant and tighten the locking nut. Verify that the milling guide is centered on the midline. To ensure construct stability, place the sharp orientation pin through the superior hole in the milling guide and manually drive it into the bone under fluoroscopy control (Fig. 154-4).
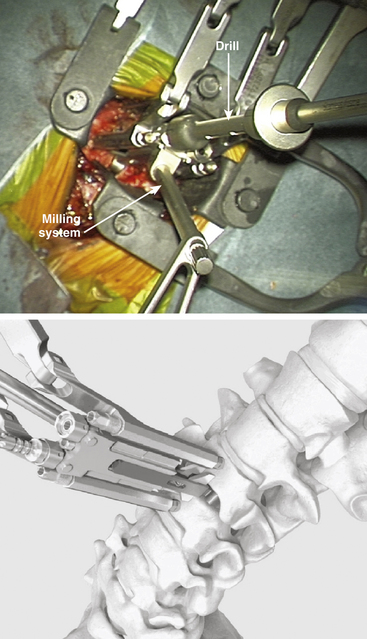
FIGURE 154-3 The trial implant is inserted under x-ray control. The milling system is loaded onto the trial implant.
(A and B courtesy of Mr. A. Saxena.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree