Chapter 75 Disorders of Nerve Roots and Plexuses
Disorders of Nerve Roots
Anatomical Features
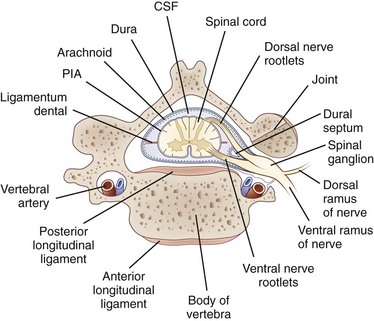
Each nerve root is attached to the spinal cord by four to eight rootlets that are splayed out in a longitudinal direction (Rankine, 2004). The dorsal roots are attached to the spinal cord at a well-defined posterolateral sulcus. The ventral rootlets are more widely separated and emerge over a greater area. At each spinal cord segment, a pair of dorsal and ventral roots unite just beyond the DRG to form a short mixed spinal nerve that divides into a thin dorsal ramus and a thicker ventral ramus (Fig. 75.1). The dorsal ramus innervates the deep posterior muscles of the neck and trunk (the paraspinal muscles) and the skin overlying these areas. The ventral ramus (the large anterior branch) contributes to the intercostal nerve or the cervical, brachial, or lumbosacral plexus and thereby supplies the trunk and limb muscles.
The nerve roots lie freely in the subarachnoid space, covered by a thin root sheath which is a layer of flattened cells continuous with the pial and arachnoidal coverings of the spinal cord. They lack the epineurial and perineurial coverings found in peripheral nerves. Compared with spinal nerves, the roots have many fewer connective tissue cells in the endoneurium and considerably less collagen. A capillary network derived from the radicular arteries provides the blood supply to the spinal nerve roots (Levin, 2002).
Where the nerve roots form the mixed spinal nerve, the pial covering of the root becomes continuous with spinal nerve perineurium, and the nerve takes the dural nerve root sheath through the intervertebral foramen to become continuous with the epineurium of the mixed nerve. At the intervertebral foramen, the root-DRG-spinal nerve complex is securely attached by a fibrous sheath to the transverse process of the vertebral body. In general, the DRG is located in a protected position within the intervertebral foramina, but at the lumbar and sacral levels, the DRG resides proximal to the neural foramina in an intraspinal location (Levin, 2002). There they may be vulnerable to disk herniation and the complications of osteoarthritis and lumbosacral spondylosis.
The dorsal roots contain sensory fibers that are central processes of the unipolar neurons of the DRG. On reaching the spinal cord, these fibers either synapse with other neurons in the posterior horn or pass directly into the posterior columns. In the ventral root, most fibers are essentially direct extensions of anterior horn motor neurons (alpha, beta, and gamma fibers) or of neurons in the intermediolateral horn (preganglionic sympathetic neurons found in lower cervical and thoracic segments). In addition, ventral roots contain a population of unmyelinated and thinly myelinated axons that come from sensory and sympathetic ganglia (Hildebrand et al., 1997).
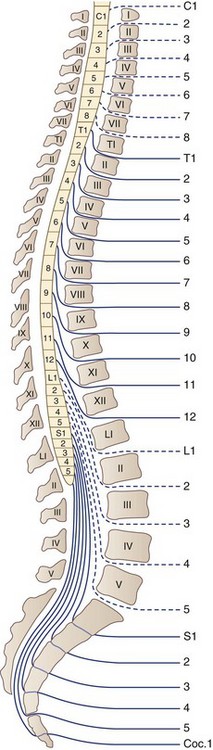
There are 31 pairs of spinal nerves that run through the intervertebral foramina of the vertebral column: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal (Fig. 75.2). A feature of clinical relevance is the pattern formed by the lumbar and sacral roots as they leave the spinal cord and make their way to their respective DRG to form spinal nerves. In the adult, the spinal cord is shorter than the spinal column, ending usually between L1 and L2. Therefore, the lumbar and sacral roots descend caudally from the spinal cord to reach the individual intervertebral foramina, forming the cauda equina. The concentration of so many nerve roots in a confined area makes this structure vulnerable to a range of pathological processes.
Traumatic Radiculopathies
Nerve Root Avulsion
The spinal roots have approximately one-tenth the tensile strength of the peripheral nerves because of lesser amounts of collagen and the absence of epineurial and perineurial sheaths in the roots. Therefore, the nerve roots are the weak link in the nerve root–spinal nerve–plexus complex, and nerve root avulsion from the spinal cord typically results from a severe traction injury affecting the upper limb. Ventral roots are more vulnerable to avulsion than dorsal roots, a consequence of the dorsal roots having the interposed DRG and a thicker dural sheath. In most cases, root avulsion occurs in the cervical region. Lumbosacral nerve root avulsions are rare, with only 35 cases reported between 1955 and 1996, and when they occur are generally associated with fractures of the sacroiliac joint with diastasis of the symphysis pubis or fractures of the pubic rami (Chin and Chew, 1997).
Avulsion at the level of the cervical roots can be total, as for a motorcyclist injury, or can result in two clinical syndromes of partial avulsion. One is Erb-Duchenne palsy, in which the arm hangs at the side internally rotated and extended at the elbow because of paralysis of C5- and C6-innervated muscles (the supraspinatus and infraspinatus, deltoid, biceps). The second is Dejerine-Klumpke palsy, in which there is weakness and wasting of the intrinsic hand muscles, with a characteristic clawhand deformity due to paralysis of C8- and T1-innervated muscles. Injuries responsible for Erb-Duchenne palsy are those that cause a sudden and severe increase in the angle between the neck and shoulder, generating stresses that are readily transmitted in the direct line along the upper portion of the brachial plexus to the C5 and C6 roots. Today, motorcycle accidents are the most common causes of this injury, but the C5 and C6 root avulsions classically occurred in the newborn during obstetrical procedures. Brachial plexus injuries in the newborn are discussed in Chapter 80. Dejerine-Klumpke palsy occurs when the limb is elevated beyond 90 degrees and tension falls directly on the lower trunk of the plexus, C8, and T1 roots. Such an injury may occur in a fall from a height in which the outstretched arm grasps an object to arrest the fall, leading to severe stretching of the C7, C8, and T1 roots, or during obstetrical traction on the extended arm when delivering the baby arm first.
Clinical Features and Diagnosis
Electrophysiological tests include the measurement of a sensory nerve action potential (SNAP) and needle EMG examination of the cervical paraspinal muscles. In the setting of an isolated C5 root avulsion, the SNAP should be preserved despite complete anesthesia in the dermatome, because the peripheral axons and the DRG cell bodies remain intact. Needle EMG of the cervical paraspinal muscles permits separation of damage of the plexus and of ventral root fibers because the posterior primary ramus, which arises just beyond the DRG and proximal to the plexus as the first branch of the spinal nerve, innervates these muscles (see Fig. 75.1). Thus, cervical paraspinal fibrillation potentials support the diagnosis of root avulsion. Paraspinal muscles have also been evaluated radiologically in the setting of root avulsion. Contrast-enhanced MRI studies of the cervical paraspinal muscles showing severe atrophy were accurate in indicating root avulsion injuries, and abnormal enhancement in the multifidus muscle was the most accurate among paraspinal muscle findings (Hayashi et al., 2002). Intraspinal neuroimaging using postmyelographic CT or MRI usually demonstrates an outpouching of the dura filled with contrast or CSF at the level of the avulsed root (Hayashi et al., 1998). This posttraumatic meningocele results from tears in the dura and arachnoid sustained during root avulsion. Advances in MRI technology now provide high-resolution images that can demonstrate root avulsion and obviate the need for CT myelography (Rankine, 2004).
Treatment
Root avulsion produces a severe neurological deficit that was considered an untreatable injury up until a few decades ago. In the past 30 years, microsurgical techniques and intraoperative electrophysiological studies have improved prospects for recovery for many patients with severe injury to peripheral nerves. The procedures of neurolysis (freeing intact nerve from scar tissue), nerve grafting (bridging ruptured nerves), and neurotization, or nerve transfer (attaching a donor nerve to the ruptured distal stump), have all been employed in the management of root avulsion injuries (Rankine, 2004). After C5 and C6 root avulsion injuries, for example, the plegic elbow flexors may be restored by several procedures that provide for neurotization of the musculocutaneous nerve, including reinnervating the biceps with an ulnar nerve fascicle (Tung et al., 2003) or employing a branch of the accessory nerve using a sural nerve graft (Samil et al., 2003); intercostal and phrenic nerves have also been used (Rankine, 2004). Carlstedt and colleagues (1995) pioneered another approach—nerve root repair and reimplantation. They reported on a patient who had an avulsion injury involving C6-T1 in whom they were able to successfully implant two ventral roots (C6 directly and C7 via sural nerve grafts) into the spinal cord through slits in the pia mater. The surgical treatment of patients with avulsion injuries is an area of active ongoing investigation with the promise that if continuity between spinal cord and nerve roots can be restored, subsequent recovery of function may be possible (Fournier et al., 2001). The sometimes intractable pain of cervical root avulsion injuries may be successfully treated with dorsal root entry zone coagulation procedures (Samii et al., 2001).
Disk Herniation
Beginning in the third or fourth decade of life, cervical and lumbar intervertebral disks are liable to herniate into the spinal canal or intervertebral foramina and impinge on the spinal cord (in the case of cervical disk herniations), nerve roots (in both cervical and lumbosacral regions), or both (at the cervical level where on occasion large central and paracentral disk herniations may produce a myeloradiculopathy) (see Chapter 73).
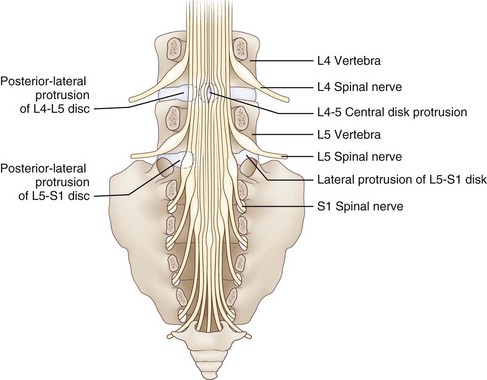
Reinforcing the annulus fibrosus posteriorly is the posterior longitudinal ligament, which in the lumbar region is dense and strong centrally and less well developed in its lateral portion. Because of this anatomical feature, the direction of lumbar disk herniations tends to be posterolateral, compressing the nerve roots in the lateral recess of the spinal canal. Less commonly, more lateral (foraminal) herniations compress the nerve root against the vertebral pedicle in the intervertebral foramen (Fig. 75.3). On occasion, the degenerative process may be particularly severe. This leads to large rents in the annulus and posterior longitudinal ligament, thereby permitting disk material to herniate into the spinal canal as a free fragment with the potentially damaging capacity to migrate superiorly or inferiorly and compress two or more nerve roots of the cauda equina. Most cervical disk herniations are posterolateral or foraminal.
In the cervical and lumbar regions, alteration in the integrity of the disk space is a component of a degenerative condition termed spondylosis, characterized by osteoarthritic changes in the joints of the spine, the disk per se (desiccation and shrinkage of the normally semisolid, gelatinous nucleus pulposus), and the facet joints. Immunohistochemical examination of herniated cervical disks points to an inflammatory process associated with neovascularization and increased expression of matrix metalloproteinase and inducible nitric oxide (NO) synthetase (Furusawa et al., 2001). The release of NO by disk cells may contribute to the process of disk degeneration by inducing apoptosis (Kohyama et al., 2000). Because it spawns osteophyte formation, spondylosis leads to compromise of the spinal cord in the spinal canal and the nerve roots in the intervertebral foramina. Restriction in the dimensions of these bony canals may be exacerbated by thickening and hypertrophy of the ligamentum flavum, which is especially detrimental in patients with congenital cervical or lumbar canal stenosis.
Clinical Features
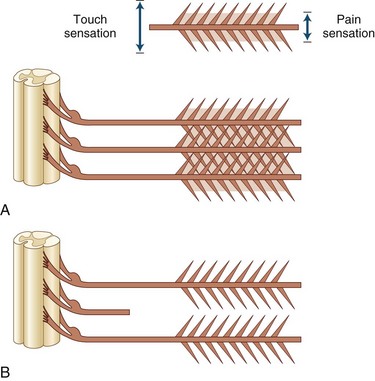
Root compression from disk herniation gives rise to a distinctive clinical syndrome that in its fully developed form comprises radicular pain, dermatomal sensory loss, weakness in the myotome, and reduction or loss of the deep tendon reflex subserved by the affected root (Carette and Fehlings, 2005). Nerve root pain is variably described as knifelike or aching and is widely distributed, projecting to the sclerotome (defined as deep structures such as muscles and bones innervated by the root). Typically, root pain is aggravated by coughing, sneezing, and straining at stool (actions that require a Valsalva maneuver and raise intraspinal pressure). Accompanying the pain are paresthesias referred to the specific dermatome, especially to the distal regions of the dermatomes; indeed, these sensations strongly suggest that the pain has its origins in compressed nerve roots rather than spondylotic facet joints. Sensory loss caused by the compromise of a single root may be difficult to ascertain because of the overlapping territories of adjacent roots, although loss of pain is usually more easily demonstrated than loss of light touch sensation (Fig. 75.4).
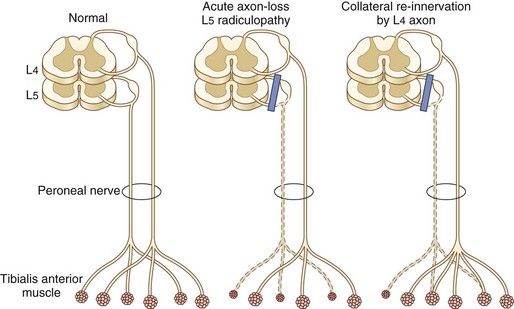
In the lumbosacral region, 95% of disk herniations occur at the L4-L5 or L5-S1 levels; L3-L4 and higher lumbar disk herniations are uncommon (Deyo and Weinstein, 2001). Knowing that the L4 root exits beneath the pedicle of L4 through the L4-L5 foramen and that L5 exits through the L5-S1 foramen, one might predict that disk herniation at these levels would generally compress the L4 and L5 roots, respectively (see Fig. 75.3). In perhaps only 10% of cases of the disk herniating far laterally into the foramen is there compression of the exiting nerve root. More commonly, the posterolateral disk herniation compresses the nerve root passing through the foramen below that disk, so L4-L5 and L5-S1 herniations usually produce L5 and S1 radiculopathies, respectively.
In the cervical region, it is likely that the greater mobility at levels C5-C6 and C6-C7 promotes the development of cervical disk degeneration with annulus fraying and subsequent disk protrusion. Cervical nerve roots emerge above the vertebra that shares the same numerical designation. Therefore C7 exits between C6 and C7, and spondylotic changes with or without additional acute disk herniation would be expected to compress the C7 nerve root. Similarly, disk protrusion at C5-C6 and C7-T1 would compress the C6 and C8 roots, respectively. In the classic study of Yoss and associates in 1957, clinical and radiological evidence of radiculopathy was found to occur most often at C7 (70%), less frequently at C6 (19%-25%), uncommonly at C8 (4%-10%) and C5 (2%). Radiculopathy involving the T1 root is a clinical rarity (Levin, 1999).
Diagnosis
Diagnosis is aided by a variety of imaging techniques (e.g., plain radiography, myelography, CT myelography, MRI) and EMG testing (Carette and Fehlings, 2005) (see Chapter 73). Both diagnostic modalities—the imaging approach that reveals anatomical details and the EMG techniques that disclose neurophysiological function—agree in the majority of patients (60%) with a clinical history compatible with cervical or lumbosacral radiculopathy, although only the results of one study will be positive in a significant minority of patients (40%) (Nardin et al., 1999). Although plain radiography is unhelpful in the identification of a herniated disk per se, in both the cervical and the lumbar area, it reveals spondylotic changes when present. It also may be useful for identifying less common disorders that produce radicular symptoms and signs: bony metastases, infection, fracture, and spondylolisthesis, for example.
In the cervical region, the best methods for assessing the relationship between neural structures (spinal cord and nerve root) and their fibro-osseous surroundings (disk, spinal canal, and foramen) are postmyelography CT (unenhanced CT reveals little more than the presence of bony changes) and MRI. MRI is equivalent in diagnostic capacity to postmyelography CT and therefore is preferred. In the lumbosacral region, CT is an effective method for evaluating disk disease, but when available, MRI is considered the superior imaging study. Its excellent resolution, multiplanar imaging, the ability to see the entire lumbar spine including the conus, and the absence of ionizing radiation make it highly sensitive in detecting structural radicular disorders (Ashkan et al., 2002).
A variety of neurophysiological tests are used to assess patients with disk herniation: motor and sensory nerve conduction studies, late responses, somatosensory evoked potentials, nerve root stimulation, and needle electrode examination. Sensory conduction studies are useful in the evaluation of a patient suspected of radiculopathy because SNAPs are typically normal (because the lesion is rostral to the DRG in the intervertebral foramina) even in the face of clinical sensory loss, in contrast to the situation in plexopathy and peripheral nerve trunk lesions, where SNAPs are attenuated or absent. In the specific instance of L5 radiculopathy, however, because the L5 DRG may reside proximal to the neural foramen, if intraspinal pathology is severe enough, compression of the L5 DRG may lead to loss of the superficial peroneal nerve SNAP (Levin, 1998).
Needle EMG is the most useful electrodiagnostic procedure in the diagnosis of suspected radiculopathy (Wilbourn and Aminoff, 1998). A study is considered positive if abnormalities—especially acute changes of denervation including fibrillation potentials and positive sharp waves—are present in two or more muscles that receive innervation from the same root, preferably via different peripheral nerves. No abnormalities should be detected in muscles innervated by the affected root’s rostral and caudal neighbors. Reduced motor unit potential (MUP) recruitment (manifested by decreased numbers of MUPs firing at an increased rate) and MUP abnormalities of reinnervation (high-amplitude, increased duration, polyphasic MUPs) are also sought by the needle electrode but are not as reliable as fibrillation potentials in establishing a definitive diagnosis of radiculopathy. Absence of fibrillation potentials does not, however, exclude the diagnosis of radiculopathy. Two main reasons for this exist. First, examination in the first 1 to 3 weeks after onset of nerve root compromise may be negative because it takes approximately 2 weeks for these potentials to appear. At the early stages in the process of nerve root compression, the only needle electrode examination manifestation of radiculopathy might be reduced MUP recruitment resulting from either axon loss, conduction block, or both. Second, fibrillation potentials disappear as denervated fibers are reinnervated by axons of the same or an adjacent myotome beginning 2 to 3 months after nerve root compression (Fig. 75.5). Thus in the later phases of nerve root compression, the only needle EMG changes indicative of radiculopathy might be chronic neurogenic changes of reduced recruitment and MUP remodeling. The distribution of fibrillation potentials is relatively stereotyped for C5, C7, and C8 radiculopathies, whereas C6 radiculopathy has the most variable presentation. In about half of patients, the findings are similar to C5 radiculopathy, whereas in the other half, findings are identical to C7 radiculopathy (Levin et al., 1996). A patient with the uncommon disk compression of T1 was found to have isolated fibrillation potential activity of the abductor pollicis brevis (Levin, 1999).
Treatment
For cervical disk protrusion and spondylotic radiculopathy, the mainstay of treatment is conservative management—a combination of a period of reduced physical activity with use of a soft cervical collar, physiotherapy, and antiinflammatory and analgesic agents. Most patients improve, even those with mild to moderate motor deficits. Indeed, in some cases, herniated cervical disks have been observed to regress on MRI images, a circumstance that appears more likely to occur if disk material has extruded and becomes exposed to the epidural space (Mochida et al., 1998). Although there appears to be a short-term benefit to surgical decompression of an affected nerve root with regard to pain, weakness, or sensory loss, at 1 year, there is no significant difference between the outcome of surgical or conservative management (physical therapy or hard cervical collar immobilization) (Fouyas et al., 2002). A surgical approach may be warranted, however, in selected cases: (1) if there is unremitting pain despite an adequate trial of conservative management, (2) if there is progressive weakness in the territory of the compromised nerve root, or (3) if there are clinical and radiological signs of an accompanying new onset of myelopathy, although in a group of patients with mild or moderate myelopathy, those managed surgically had the same outcome (degree of functional disability) as those allocated to conservative treatment (Fouyas et al., 2002).
In the lumbosacral region, disk herniation and spondylotic changes respond to conservative management in more than 90% of patients. Bed rest had been recommended as the centerpiece of patient care, but controlled trials have demonstrated that back-strengthening exercises under the direction of a physical therapist, performed within the limits of the patient’s pain, result in more rapid resolution of pain and return to normal function (Vroomen et al., 1999). Follow-up MRI studies in conservatively managed patients indicate reduction in size or disappearance of herniated nucleus pulposus corresponding to improvement in clinical findings (Komori et al., 1996). Epidural corticosteroid injection may help relieve pain but does not improve neurological function or reduce the need for surgery (Carette et al., 1997). A single intravenous (IV) bolus of methylprednisolone (500 mg) given to patients with acute diskogenic sciatica of less than 6 weeks’ duration provided short-term improvement in leg pain, but the effect was relatively small, with no effect on functional disability, and transitory (3 days) (Finckh et al., 2006). When a patient population with sciatica due to a herniated lumbar disk is followed at regular intervals for more than 10 years, surgically treated patients report more complete relief of leg pain and improved function and satisfaction compared with the nonsurgically treated group. However, improvement in the patient’s predominant symptom and work or disability outcomes were similar in the two groups (Atlas et al., 2005). Three situations occur in which surgical referral is indicated: (1) in patients presenting with cauda equina syndrome, for which surgery may be required urgently, (2) if the neurological deficit is severe or progressing, or (3) if severe radicular pain continues after 4 to 6 weeks of conservative management.
Diabetic Polyradiculoneuropathy
When there is predominant involvement of the thoracic roots, the presenting symptoms are generally pain and paresthesias of rapid onset in the abdominal and chest wall. The trunk pain may be severe, described variably as burning, sharp, aching, and throbbing. It may mimic the pain of acute cardiac or intraabdominal medical emergencies and may simulate disk disease, but the rarity of thoracic disk protrusions and the usual development of a myelopathy help exclude this diagnosis. Findings of diabetic thoracoabdominal polyradiculoneuropathy include heightened sensitivity to light touch over affected regions; patches of sensory loss on the anterior, lateral, or posterior aspects of the trunk; and unilateral abdominal swelling due to localized weakness of the abdominal wall muscles (Longstreth, 1997).
Diabetic lumbosacral polyradiculoneuropathy involves the legs, especially the anterior thighs, with pain, dysesthesia, and weakness, reflecting the major involvement of upper lumbar roots. A variety of names have been used to describe it, including diabetic amyotrophy, proximal diabetic neuropathy, diabetic lumbosacral plexopathy, diabetic femoral neuropathy, and Bruns-Garland syndrome. Because it is likely that the brunt of nerve pathology falls on the nerve roots, it can be designated as diabetic polyradiculoneuropathy. Motor, sensory, and autonomic fibers are all affected by the disease process (Dyck et al., 1999).
In most patients, onset is fairly abrupt, with symptoms developing over days to a couple of weeks. Early in the course of the condition, the clinical findings are usually unilateral and include weakness of muscles supplied by L2-L4 roots (iliopsoas, quadriceps, and hip adductors), reduced or absent patellar reflex, and mild impairment of sensation over the anterior thigh. As time passes, there may be territorial spread, a term used by Bastron and Thomas in 1981 to describe proximal, distal, or contralateral involvement as the polyradiculoneuropathy evolves. Worsening may occur in a steady or a stepwise fashion, and it may take several weeks to progress from onset to peak of the disease. At its peak, weakness varies in severity and extent from a mildly affected patient with slight unilateral thigh weakness to a profound degree of bilateral leg weakness in the territory of the L2-S2 nerve roots. Upper-extremity involvement appears to occur in approximately 15% of patients with diabetic lumbosacral radicular plexopathy as a unilateral or asymmetrical sensorimotor neuropathy that primarily affects hands and forearms. Like the lumbosacral syndrome, EMG findings suggest a multifocal axon-loss process localized to roots, plexus, or peripheral nerve (Katz et al., 2001). Rarely, the process of territorial spread is so extensive that it involves a multiplicity of nerve roots along the entire spinal cord and leads to profound generalized weakness, a condition designated diabetic cachexia.
Laboratory studies disclose elevated fasting blood glucose in the vast majority of patients; when values are normal, they are found in treated diabetics. The erythrocyte sedimentation rate is usually normal, but in a subgroup of patients with diabetic lumbosacral polyradiculoneuropathy, it is elevated, a clue perhaps to an immune-mediated pathogenesis. The typical electrodiagnostic findings comprise features of a sensorimotor axon-loss polyneuropathy (diminished sensory and motor action potentials, normal or slightly prolonged distal latencies, and normal or mildly slowed conduction velocities) with additional needle electrode examination findings of active and chronic denervation changes in paraspinal, pelvic-girdle, and thigh muscles. Taken together, the findings reflect multifocal axonal damage to the nerve roots and lumbosacral plexus (Amato and Barohn, 2001). Although clinical findings may point to unilateral involvement, the electrodiagnostic examination generally discloses bilateral signs. Imaging studies of the thoracic and lumbosacral spinal canal with CT, myelography, and MRI are typically normal but are almost always necessary to exclude a structural abnormality of the nerve roots that may simulate diabetic polyradiculopathy. The CSF protein level is usually increased to an average of 120 mg/dL, but in some patients values exceed 350 mg/dL; pleocytosis is not a feature of this condition. Biopsy of proximal nerve sensory branches reveals axon loss and demyelination; in more severely affected patients, inflammatory cell infiltration and vasculitis is found (Said et al., 1997). Further studies of nerve biopsy specimens indicate that a microscopic vasculitis (involvement of small arterioles, venules, and capillaries) leads to ischemic injury, which in turn causes axonal degeneration and secondary segmental demyelination (Dyck et al., 1999). The presence of a small-vessel vasculitis with distinctive pathological features including transmural polymorphonuclear leukocyte infiltration of postcapillary venules and endothelial deposits of immunoglobulin (Ig)M and activated complement supports an immune-mediated inflammatory pathogenesis for this disorder (Kelkar et al., 2000).
Electrophysiological studies have suggested that a demyelinating polyneuropathy indistinguishable from chronic inflammatory demyelinating polyneuropathy (CIDP) occurs frequently in diabetes and may be the cause of a severe motor sensory polyneuropathy, sometimes with features of a plexopathy (Sharma et al., 2002a, 2002b).
Therapy is usually directed toward ameliorating the severe pain of this condition. The tricyclics, especially nortriptyline (with a better side-effect profile than amitriptyline), selective serotonin reuptake inhibitors (e.g., sertraline, nefazodone hydrochloride), anticonvulsants (e.g., gabapentin, carbamazepine), clonazepam, baclofen, clonidine, mexiletine, IV lidocaine, and topical capsaicin may have a role separately or in combination. Histopathological findings indicative of an immune-mediated pathogenesis have led to treatment of selected patients with intravenous immunoglobulin (IVIG) or immunosuppressive treatment or both (Krendel et al., 1995; Younger et al., 1998). Although immunotherapy may be beneficial, spontaneous improvement in some patients with painful proximal diabetic neuropathy with different patterns of inflammatory nerve lesions has been described (Said et al., 1997). A comprehensive and critical review of the literature on the role of immunotherapy of diabetic polyradiculopathy concludes that treatment remains controversial because the natural history is for spontaneous improvement, the side effects of immunotherapy may be significant, and information on efficacy is lacking from controlled clinical trials (Amato and Barohn, 2001). Prospective studies have suggested a role for immunotherapy in the treatment plan of diabetic polyradiculoneuropathy where electrophysiological findings are those of CIDP (Sharma et al., 2002a, 2002b), although the degree of improvement has been shown not to be as robust as in the immunotherapy of idiopathic CIDP (Gorson et al., 2000).
Neoplastic Polyradiculoneuropathy (Neoplastic Meningitis)
A wide variety of neoplasms are known to spread to the leptomeninges. These include solid tumors (carcinoma of the breast and lung and melanoma), non-Hodgkin lymphomas, leukemias, and intravascular lymphomatosis (Viali et al., 2000). Although neoplastic polyradiculoneuropathy usually occurs in patients known to have an underlying neoplasm, meningeal symptoms may be the first manifestation of malignancy. Neoplastic meningitis occurs in approximately 5% of all patients with cancer (Chamberlain, 2006). The clinical features of neoplastic polyradiculoneuropathy include radicular pain, dermatomal sensory loss, areflexia, weakness of the lower motor neuron type and bowel/bladder dysfunction (Balm and Hammack, 1996; Mammoser and Groves, 2010). Often the distribution of the sensory and motor deficits is widespread and simulates a severe sensorimotor polyneuropathy. Associated clinical manifestations (e.g., nuchal rigidity, confusion, cranial polyneuropathies) result from infiltration of the meninges.
At postmortem examination, the cauda equina shows discrete nodules or focal granularity (Fig. 75.6). Microscopy discloses spinal roots encased by tumor cells, which appear to infiltrate the root. Malignant cells have the capacity to penetrate the pial membrane and invade the spinal nerves (Mammoser and Groves, 2010). It is presumed that disturbed nerve root function results from several mechanisms including nerve fiber compression and ischemia.
The most revealing diagnostic procedure is lumbar puncture, which is almost always abnormal, disclosing one or more or the following: mononuclear pleocytosis, reduced CSF glucose, elevated protein, and neoplastic cells (Grossman and Krabak, 1999). Spinal fluid cytological analysis is, however, persistently negative in up to a third of patients who have compelling evidence of leptomeningeal carcinomatosis (Kim and Glantz, 2001). A sensitive electrophysiological indicator of nerve root involvement is a change in the F wave. In the symptomatic patient with cancer, prolonged F-wave latencies or absent F responses should raise suspicion of leptomeningeal metastases. Postmyelography CT adds strong evidence in support of the diagnosis if it demonstrates multiple nodular defects on the nerve roots, but spinal MRI, especially with gadolinium enhancement, is the initial test of choice in the cancer patient in whom leptomeningeal involvement of the spine is suspected (Gleissner and Chamberlain, 2006). Approximately 50% of patients with neoplastic meningitis and spinal symptoms have abnormalities on these studies. Gadolinium-enhanced MRI of the brain discloses abnormalities, including contrast enhancement of the basilar cisterns or cortical convexities and hydrocephalus.
Standard therapy for neoplastic meningitis is essentially palliative; it does, however, afford stabilization and protection from further neurological deterioration (Chamberlain, 2006). A multidisciplinary approach is recommended, with input from medical oncology, neuro-oncology, radiation oncology, and neurosurgery (Mammoser and Groves, 2010). With treatment that includes radiotherapy to sites of symptomatic disease, intrathecal or intraventricular chemotherapy (methotrexate, thiotepa, and cytosine arabinoside), and optimal management of the underlying malignancy, median survivals of 3 to 6 months may be achieved (Chamberlain, 2006; Kim and Glantz, 2001). On occasion, longer-term survival is observed in patients with neoplastic meningitis accompanying breast cancer (13% survival rate at 1 year and 6% at 2 years), melanoma, and lymphoma (Jaeckle, 2006). A complication of aggressive treatment is a necrotizing leukoencephalopathy that becomes symptomatic months after treatment with radiation and intrathecal methotrexate (Grossman and Krabak, 1999).
Infectious Radiculopathy
Tabes Dorsalis
Tabes dorsalis, the most common form of late neurosyphilis, begins as a spirochetal (Treponema pallidum) meningitis (see Chapter 53C). After 10 to 20 years of persistent infection, damage to the dorsal roots is severe and extensive, producing a set of characteristic symptoms and signs. Symptoms are lightning pains, ataxia, and bladder disturbance; signs are Argyll Robertson pupils, areflexia, loss of proprioceptive sense, Charcot joints, and trophic ulcers. Lancinating or lightning pains are brief, sharp, and stabbing; they are more apt to occur in the legs than elsewhere. Sensory disturbances such as coldness, numbness, and tingling also occur and are associated with impairment of light touch, pain, and thermal sensation. Sudden visceral crises, characterized by the abrupt onset of epigastric pain that spreads around the body or up over the chest, occur in some 20% of patients.
Most of the features of tabes dorsalis can be explained by lesions of the dorsal roots, dorsal root ganglia, and posterior columns (Gilad et al., 2007). Ataxia is due to the destruction of proprioceptive fibers, insensitivity to pain follows partial loss of small myelinated and unmyelinated fibers, and bladder hypotonia with overflow incontinence, constipation, and impotence is the result of sacral root damage. Pathological study discloses thinning and grayness of the posterior roots, especially in the lumbosacral region, and the spinal cord shows degeneration of the posterior columns. A mild reduction of neurons in the DRG occurs, and there is little change in the peripheral nerves. Inflammation may occur all along the posterior root.
Polyradiculoneuropathy in Human Immunodeficiency Virus–Infected Patients
Cytomegalovirus (CMV) polyradiculoneuropathy is a rapidly progressive opportunistic infection that usually occurs late in the course of human immunodeficiency virus (HIV) infection when the CD4 count is very low (<200 cells/mL) and acquired immunodeficiency syndrome (AIDS)-defining infections are present. Uncommonly, it is the initial manifestation of AIDS (Anders and Goebel, 1998). Patients often have evidence of systemic CMV infection (retinitis, gastroenteritis). The presentation is marked by rapid onset of pain and paresthesias in the legs and perineal region, associated with urinary retention and progressive ascending weakness of the lower extremities (Robinson-Papp and Simpson, 2009). Examination discloses a severe cauda equina syndrome, the combination of flaccid paraparesis, absent lower-limb deep tendon reflexes, reduced or absent sphincter tone, and variable loss of light touch, vibration, and joint position sense. The upper extremities and cranial nerves may be involved in advanced cases (Robinson-Papp and Simpson, 2009).
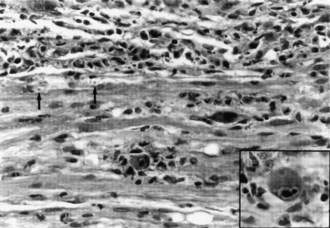
A gadolinium-enhanced MRI of the lumbosacral spine is necessary to exclude a compressive lesion of the cauda equina and is generally the first study performed (Robinson-Papp and Simpson, 2009). The CSF has an elevated protein level, depressed glucose level, polymorphonuclear pleocytosis, and a positive CMV polymerase chain reaction (PCR) (Anders and Goebel, 1998). CMV may be isolated from CSF cultures. The needle EMG discloses widespread fibrillation potentials in lower-extremity muscles, and sensory conduction studies may reveal an associated distal sensory neuropathy that is common in the late stages of HIV infection. MRI of the lumbosacral region is usually normal, but adhesive arachnoiditis has been described. The pathological features are marked inflammation and extensive necrosis of dorsal and ventral roots. Cytomegalic inclusions may be found in the nucleus and cytoplasm of endothelial and Schwann cells (Fig. 75.7).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree