Chapter 77 Disorders of the Autonomic Nervous System
Classification of Autonomic Disorders
Clinical Features of Autonomic Impairment
Assessment of Autonomic Function
Functional Autonomic Disorders
Autonomic Disorders Characterized by Excessive Autonomic Outflow
Predominantly Peripheral Afferent Structural Autonomic Disorders Characterized by Impaired Autonomic Outflow
Predominantly Peripheral Efferent Structural Autonomic Disorders Characterized by Impaired Autonomic Outflow
Predominantly Central Structural Autonomic Disorders Characterized by Impaired Autonomic Outflow
Nonautonomic Disorders Causing Hypotension or Syncope to Consider in the Differential Diagnosis
At the higher control levels in the brain, autonomic integrators and signals are integrated and expressed subconsciously through the central autonomic network (Benarroch, 1997). This overlies a strong circadian rhythm of autonomic function. The autonomic nervous system consists of two large divisions, the sympathetic (thoracolumbar) outflow, and the parasympathetic (craniosacral) outflow. The two divisions are defined by their anatomical origin rather than by their physiological characteristics. The circadian rhythm of autonomic function originates in the suprachiasmatic nucleus and is conveyed to the hypothalamus and brainstem. Light falling on retinal ganglion cell dendrites (not rods or cones) in the eye and transmitted via the retinohypothalamic tract entrains this rhythm. Other key inputs to autonomic outflow originate in the insular cortex and the amygdala. The principal integration of autonomic outflow to the cardiovascular system lies in the medulla. Stretch-sensitive baroreflex mechanoreceptors in the blood vessels of the thorax and neck relay information about blood pressure and blood volume through the glossopharyngeal (from carotid arteries) and vagus (from aorta) nerves to the nucleus tractus solitarius (NTS) in the posterior medulla. Excitatory neurons from the NTS innervate the dorsal motor nucleus of the vagus, which regulates parasympathetic outflow. Inhibitory neurons project to areas in the ventrolateral medulla from which sympathetic outflow is regulated. The most important such site is the rostral ventrolateral medulla.
The autonomic nervous system exerts widespread control over homeostasis (Goldstein, 2001; Mathias and Bannister, 2002). Almost every organ system receives regulatory information from the central nervous system (CNS) through the autonomic efferents (Fig. 77.1), and increasingly we recognize that afferent input into the central autonomic network regulates not only the output of the autonomic system but also much CNS function not generally considered to be autonomic in nature. The emerging concept is pervasive integration of autonomic activities with brain and body.
Classification of Autonomic Disorders
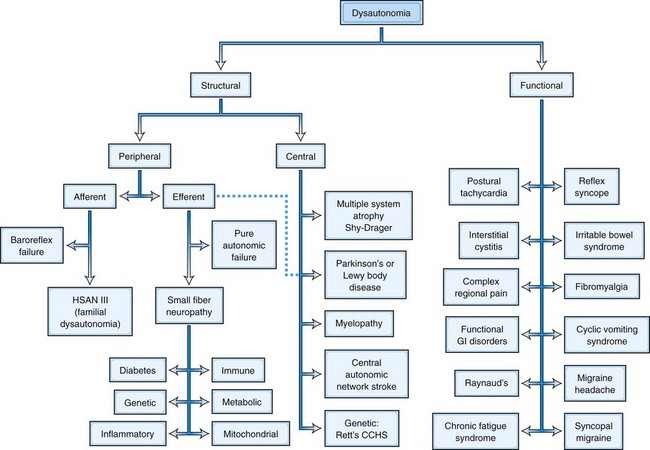
Since by definition, structural disorders have a known pathological substrate, they can be divided into two camps, those that involve the peripheral nervous system and those that involve central pathways. Such a division would be more challenging in functional disorders, where a specific etiopathology is generally not defined, with certain rare exceptions (see later discussion and Fig. 77.1). Peripheral disorders can be further subdivided by whether the dominant pathological process involves afferent or efferent fibers. As can be discerned from Fig. 77.1, most peripheral disorders involve efferent nerves. It should be kept in mind that few if any of the structural disorders are “pure” either in their central versus peripheral localization or in afferent versus efferent functional classification. For example, diabetes has been shown to involve central pathways, and PD involves peripheral ganglia, symbolized by the dashed line in the figure connecting that box to the efferent peripheral category.
These two basic classes of dysautonomias, structural and functional, contrast with one another in other ways as well. Patients with structural disorders, in spite of their extensive pathological changes in the nervous system, tend to harbor proportionally few symptoms; for example, the majority of patients do not realize when their systolic blood pressure drops by nearly 90 mm Hg (Arbogast et al., 2009). In contrast, patients with functional disorders harbor enormous numbers of complaints, just as disproportionate with the demonstrable pathological involvement of the autonomic nervous system but in the opposite sense (Ojha, 2011). These disorders are now being considered as multisystem rather than involving a single end-organ. Another difference relates to prognosis. Structural disorders in general carry a very poor prognosis. For example, diabetics with autonomic neuropathy have a 5-year mortality between 25% and 50% (Ewing et al., 1976). Patients with MSA survive only 7 to 9 years from onset (Schrag et al., 2008). In contrast, while functional disorders can be extremely disabling (Benrud-Larson et al., 2002; O’Leary and Sant, 1997; Spiegel et al., 2008), their prognosis for longevity is generally good.
Clinical Features of Autonomic Impairment
Cardiovascular
Normally when one stands, systolic blood pressure falls about 10 mm Hg, and diastolic pressure increases 5 mm Hg. Heart rate rises 5 to 20 beats per minute (bpm). A fall in blood pressure of 20/10 mm Hg or more in the first 3 minutes of standing defines orthostatic hypotension (Consensus Committee of the American Autonomic Society and the American Academy of Neurology, 1996). If there is not a fall in blood pressure of 20/10 on standing, but the patient is symptomatic and experiencing tachycardia, orthostatic intolerance is present, qualifying as POTS if the heart rate rise is greater than 30 bpm in the first 10 minutes in the upright position (Schondorf and Low, 1993). The most common symptoms of orthostatic hypotension are dizziness, dimming of vision, and discomfort in the neck and head. Orthostatic hypotension is greatest, and hence most easily detected, in the hour after ingesting a large breakfast. Carbohydrate acts as a depressor more than protein or fat. It is important to note that a majority of patients with orthostatic hypotension will not have the usual expected symptoms and may present with falls only (Arbogast et al., 2009), so it is vital to not rely solely on symptom reports in the elderly population.
Pulmonary
Lung function is generally preserved in autonomic failure. Patients with MSA frequently experience sleep apnea, and in consequence of these apneic spells, major perturbations in blood pressure may arise acutely. This is in part dependent on the role of carbon dioxide in determining blood pressure level in autonomic failure. Hypoventilation increases blood pressure in autonomic failure, whereas hyperventilation may lower blood pressure significantly. Patients have occasionally noted improved orthostatic tolerance while breathing through a dead space, although this has never been a recommendation in practice. Because of impaired swallowing in patients with MSA (Seppi et al., 2005), aspiration pneumonia occurs frequently and can be missed because the patient may not manifest the expected fever. With early diagnosis and treatment of aspiration pneumonia, patients often do well, returning to their prior state of health.
Gastrointestinal
Constipation occurs in many patients with autonomic failure, but patients with diabetic dysautonomia may also have frequent and often severe diarrhea as well as significant gastroparesis. The diarrhea itself can prevent adequate blood pressure control because of the associated volatility of blood volume. Special problems sometimes occur in specific dysautonomias. For example, patients with Sjögren syndrome commonly have gastroesophageal reflux and may therefore have an increased risk of esophageal carcinoma. Patients with Chagas disease may have achalasia and enlargement of the esophagus, resulting in vomiting. Achalasia is also present in the 4 “A” syndrome (Allgrove disease) characterized by alacrima, achalasia, ACTH insensitivity, and autonomic neuropathy. This syndrome has been mapped to chromosome 12q13 and produced by mutations in the AAAS gene (Handschug et al., 2001). Patients with some forms of genetic autonomic failure may have strikingly severe gastrointestinal fluid loss and bowel movements 10 or more times every day, which sometimes responds to low doses of clonidine. Postprandial angina may occur with food ingestion, usually without associated ST-T wave changes. Most patients with postprandial angina in practice probably have some degree of dysautonomia, and the depressor effect of food is most prominent in the setting of impaired autonomic reflexes. Postprandial angina tends to occur with upright posture following food (especially carbohydrates). Water intake in association with eating will usually help prevent this symptom.
The gastrointestinal dysfunction in two disorders with significant autonomic involvement, namely PD and diabetes, have been better evaluated and understood. For example, the role of diabetes in gastrointestinal dysmotility has been extensively studied. Some changes in the function of the enteric nervous system in diabetes appear to result from apoptosis of enteric neurons. Oxidative stress may play a role in the cell death process. An imbalance also exists in inhibitory and excitatory neuropeptides. These factors then result in altered gut mucosa (Chandrasekharan and Srinivasan, 2007). PD is also associated with gastrointestinal problems, affecting swallowing disorders that may lead to aspiration. Salivation is not increased or may even be decreased in these patients. From early on, individuals with PD may develop delayed gastric emptying, which later in the disease may affect jejunal absorption of levodopa. Gastric emptying of liquids is usually not delayed (Goetze et al., 2006) in PD, so alternatives when giving levodopa are a liquid solution or administering it directly into the jejunum (Jost, 2010). Constipation is also very common in this disorder and represents the most common “autonomic” manifestation of PD, affecting 70% to 80% of patients. Often, severe constipation develops before the more typical symptoms of PD are noticed (Korczyn, 1990; Jost, 2010); the cause is multifactorial. Many of the medications prescribed to treat PD, mainly anticholinergics and (perhaps more controversial) levodopa, may worsen constipation, but they are not the cause, since constipation is usually present before the diagnosis of PD is made and therefore before the onset of medications. Constipation is thought to be due to degeneration of central and peripheral parasympathetic nuclei (Jost, 2010).
Other less well-characterized dysautonomias also have gastrointestinal symptoms. Individuals with POTS complain of bloating, early satiety, nausea, pain, and alternating diarrhea and constipation (Sandroni et al., 1999). In fact, more than half of the children and adults with POTS have gastrointestinal complaints, usually reporting epigastric or lower-abdominal discomfort. Children with POTS seem to suffer nausea and vomiting more often than adults, but these findings were present in both groups (Ojha et al., 2011). Interestingly, coming from the other direction, many with functional gastrointestinal problems also have cardiovascular autonomic dysfunction, primarily sympathetic. In adults, three-eighths also had parasympathetic involvement, which was not present in the pediatric group. Neuropathy was common in both groups (Camilleri and Fealey, 1990; Chelimsky and Chelimsky, 2001). Importantly, when both a functional gastrointestinal disorder and POTS are present, the gastrointestinal symptoms may resolve with treatment of the orthostatic intolerance (Sullivan et al., 2005). The cause of the gastrointestinal symptoms in POTS in unclear and may be related to blood pooling in either the lower extremities or abdomen. Electrical activity of the stomach in POTS changes from supine to the upright position (Safder et al., 2009), suggesting either lack of accommodation or gastroparesis while upright (Buchwald et al., 1987). Cyclic vomiting syndrome (CVS) has also been associated with autonomic dysfunction, and both pediatric and adult sufferers usually have POTS associated with an autonomic neuropathy (Chelimsky and Chelimsky, 2007; Venkatesan et al., 2010).
Urinary Tract
In structural disorders, a reversal of the usual pattern of urine output occurs. Nocturia is brought on by recumbency and the attendant increase in blood pressure (Mathias et al., 2002). The weight loss during the night is often 2 to 4 pounds, and the reduction in blood volume that results partially accounts for the reduction in orthostatic tolerance seen on arising each morning. The bladder is often directly involved in dysautonomias. This autonomic involvement presents as urgency, retention, incontinence, and frequency. Urological evaluation often suggests prostatic hypertrophy in men, and surgery may be a consideration. Such surgery rarely helps patients with autonomic dysfunction and should only be considered after careful consultation between the urologist and neurologist to ascertain whether a physical obstruction is truly playing a major role. Unfortunately, the α1-antagonist class of drugs commonly used to treat prostatic hypertrophy can worsen orthostatic hypotension; conversely, the α1-agonist, midodrine, used to increase blood pressure, may occasionally increase bladder symptoms. With urine retention, urinary tract infections occur commonly. With autonomic failure, plasma renin levels are often quite low, probably because sympathetic regulation of the kidney is impaired. However, renal function is usually well preserved in most forms of autonomic failure, although not in dopamine β-hydroxylase deficiency, in which significant renal failure occurs in adulthood.
Sweating Abnormalities
Increases or decreases in sweating occur with disturbances of autonomic thermoregulatory function (Fealey, 2008; Low, 1997). Hyperhidrosis refers to conditions in which sweating is excessive for a given stimulus. It can be general or localized. General hyperhidrosis can be primary (episodic hypothermia with hyperhidrosis, or Shapiro syndrome) or secondary to other disorders. Typically, hyperhidrosis is episodic. Dramatic hyperhidrosis may occur in pheochromocytoma. Tumors may produce cytokines, which provoke fever and consequently sweating. Hyperhidrosis also occurs in powerful sympathetic excitation, such as delirium tremens or in the pressor surges of baroreflex failure.
Referral for localized hyperhidrosis (Table 77.1) is often to the neurologist. Evidence exists of enhanced sweat gland innervation coupled with increased activity of sympathetic fibers passing through T2-T4. This is especially prominent in young people. Perhaps 25% of such individuals have a positive family history of hyperhidrosis. Axillary or palmar hyperhidrosis may be so severe as to interfere with normal activities and social interactions. Several therapeutic modalities may help (Table 77.2). A major limitation in therapy of hyperhidrosis is achieving sufficient muscarinic antagonism on sweat glands without incurring unacceptable levels of muscarinic blockade elsewhere—for example, in the heart. Local application of botulinum toxin to affected skin may also be quite effective but requires repeat injections at 3- to 12-month intervals (Saadia et al., 2001). In addition, blockade of the sympathetic ganglia using pharmacological injections, radiofrequency ablations, or endoscopic gangliotomy can be very effective (Atkinson and Fealey, 2003).
Table 77.1 Pathological Hyperhidrosis Differential Diagnosis and Some Causes of Localized Hyperhidrosis
| Condition | Pathophysiological Mechanism of Sweating |
|---|---|
| Essential hyperhidrosis | |
| Perilesional and compensatory hyperhidrosis | Central and/or peripheral denervation of large numbers of sweat glands produces increased sweat secretion in those remaining innervated; often asymmetrical distribution |
| Gustatory sweating | Resprouting of secretomotor axons to supply denervated sweat glands |
| Post cerebral infarct | Loss of contralateral inhibition with cortical and upper brainstem infarction |
| Autonomic dysreflexia | Uninhibited segmental somatosympathetic reflex; recent drug prescription; includes nifedipine and sublingual captopril |
| Complex regional pain syndrome | Localized sympathetic sudomotor hyperactivity; probably axon reflex vs. direct irritation/infiltration of sympathetic preganglionic or postganglionic fibers |
| Paroxysmal localized hyperhidrosis | Myopathic: ? transiently decreased hypothalamic setpoint temperature; responsive to clonidine, a centrally acting, α2-adrenergic agonist |
Table 77.2 Treatment Measures for Primary Hyperhidrosis
| Topical Rx | 20% Aluminum chloride hexahydrate in anhydrous ethyl alcohol (Drysol). Apply half-strength to dry skin daily or every other day, mornings, and wash off. | Irritation of skin; less effective on palms and soles, which may require occlusive (plastic wrap) technique |
| Tanning Rx, iontophoresis | Glutaraldehyde (2%-10%) solution; apply 2-4 times/week as needed. | Stains skin brown; for soles of feet only |
| For palms/soles; 15-30 mA current, 20 min. at start. Drionic battery-run unit or galvanic generator needed; 3-6 treatments/week for total of 10-15 treatments initially; 1-2 treatments/week maintenance. | Shocks, tingling may occur Difficult to use in axilla Drionic unit not effective when batteries low | |
| Anticholinergic | Glycopyrrolate (Robinul/Robinul Forte) at 1-2 mg PO tid as needed; for intermittent/adjunctive treatment. | Dry mouth, blurred vision Contraindicated: glaucoma, GI tract obstruction, GU tract obstruction |
| Clonidine | Useful for paroxysmal localized (e.g., hemibody) hyperhidrosis; 0.1-0.3 mg PO tid or as TTS patch (0.1-0.3 mg/day) weekly. | Somnolence, hypotension, constipation, nausea, rash, impotence, agitation |
| Excision | Second and third thoracic ganglionic sympathectomy (palmar hyperhidrosis), sweat glands (axillary liposuction); recent preference is for T2 sympathectomy to limit compensatory hyperhidrosis. | Homer syndrome, dry skin, transient dysesthetic pain Postoperative scar or infection Compensatory hyperhidrosis of trunk, pelvis, legs, and feet |
| Botox | 50-100 mU of botulinum toxin A into each axilla or body area treated; high doses (200 mU) prolong effect; can be repeated. | Injection discomfort, variable Duration of effect 3-12 months Expensive Mild grip weakness when palm is treated Contraindicated in pregnancy, NMJ disease |
GI, Gastrointestinal; GU, genitourinary; mU, mouse units; NMJ, neuromuscular junction; PO, orally; Rx, prescription; tid, three times daily.
Adapted from Fealey, R.D., 2004. Disorders of sweating, in: Robertson, D., Biaggioni, I., Burnstock, G., et al. (Eds.), Primer on the Autonomic Nervous System, second ed. Elsevier, New York, pp. 354-357.
Idiopathic hyperhidrosis must be distinguished from compensatory hyperhidrosis due to lower body hypohidrosis, common in dysautonomias (Klein et al., 2003), which may paradoxically be described by patients as excessive sweating in the upper body. Patients who lose their ability to perspire over most of their body may preserve it in the neck and facial area and perspire disproportionately in these areas, which captures attention more than the loss of sweating elsewhere. In this setting, the hyperhidrosis actually mandates an evaluation for the reason for the anhidrosis, usually a structural dysautonomia of some type. Loss of sweating does not require specific medical therapy, but rather practical advice such as staying well hydrated, avoiding alcohol, and avoiding hot conditions. One of the most effective home remedies is a “wet shirt”: A tee shirt soaked in warm water and wrung out thoroughly before putting on provides some artificial perspiration that lasts 30 to 90 minutes in a hot environment. The associated surface cooling is striking and greatly increases the functional capacity of patients who must be in a hot environment.
Assessment of Autonomic Function
More tests of autonomic function exist than for any other neurological system. Many of these tests are readily applied at the bedside, and though easy to perform, they may be difficult to interpret in an individual patient. Most physicians who routinely follow patients with autonomic disorders develop a small armamentarium of tests that answer questions relating to the focus of their specialty. The neurologist assessing autonomic function requires tests that localize the lesion within the neuraxis and provide information about the types of fibers involved. Therefore, tests of peripheral and central sudomotor function and tests that differentiate sympathetic from parasympathetic cardiovascular function are essential. The cardiologist requires tests of blood pressure and heart rate that evaluate the mechanism of any cardiovascular dysregulation. The endocrinologist measures circulating catecholamines, corticoids, sex hormones, and rennin. These tests are centered on appreciating the hormonal impact and consequences of autonomic dysfunction (Raj et al., 2005). The ophthalmologist tests pupillary function, and the pharmacologist uses drug tests that assess normal or hypersensitive autonomic function response (Robertson et al., 2004). Despite such dramatically divergent diagnostic approaches, it is remarkable how much consensus is often achieved in terms of the actual diagnosis and therapy of an individual patient. Indeed, an interdisciplinary approach that involves multiple specialists from different disciplines may provide both broader perspective on organ-system involvement and more accurate diagnosis and may be the reason more centers are taking this direction clinically.
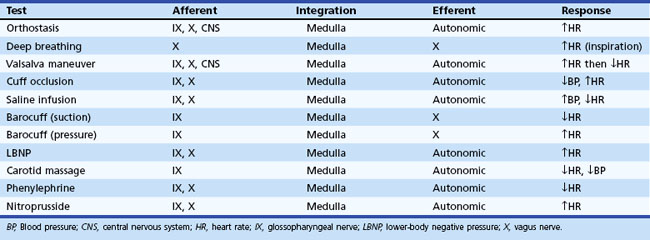
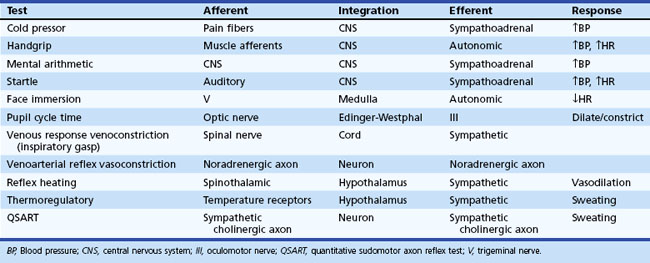
Regardless of the clinical evaluation setting, a careful history is obviously the critical diagnostic resource. A brief listing of important items in questioning patients is shown in Box 77.1. More detailed discussions of some of these may be found elsewhere. Key autonomic features in the physical examination are shown in Box 77.2. In this section, attention is given to highly informative autonomic tests. Table 77.3 displays a listing of widely employed tests of baroreflex function.
Box 77.1 Key Features in the Autonomic History
Orthostatic Test
Orthostatic symptoms are usually the most debilitating aspect of autonomic dysfunction readily amenable to therapy. For this reason, the blood pressure and heart rate response to upright posture is the starting point of any autonomic laboratory evaluation. In healthy human subjects, the cardiovascular effect of upright posture has been well defined (Low, 1997). Upon active assumption of the upright posture by standing, the vigorous contraction of large muscles leads to a transitory muscle vasodilation and a minor fall in arterial pressure for which the reflexes do not immediately compensate. However, this short-lived depressor phase is not usually seen with passive (tilt-table) upright posture. Immediately after 70- or 90-degree head-up tilt, approximately 500 mL of blood move into the veins of the legs and approximately 250 mL into the buttocks and pelvic area. A rapid vagally mediated increase in heart rate occurs, followed by a sympathetically mediated further increase. As right ventricular stroke volume declines, a depletion of blood from the pulmonary reservoir occurs, and central blood volume falls. Stroke volume falls, and cardiac output decreases about 20%. With this decline in cardiac output, blood pressure is maintained by vasoconstriction that reduces splanchnic, renal, and skeletal muscle blood flow especially, but other circulations as well.
An important aspect of evaluating responses to orthostasis is the rapid reduction in total blood volume that occurs physiologically. It is not unusual for a 12% fall in plasma volume to occur within 10 minutes of assumption of the upright posture as fluid moves from the vascular compartment into the extravascular space (Jacob et al., 2005). This accounts for the delay in appearance of symptoms in patients with mild autonomic impairment for some minutes after the actual assumption of upright posture. Therefore, the long-stand (30 minutes) test, or Schellong test, is a much more severe orthostatic stress than the short-stand (5 minutes) test commonly employed.
Sweat Testing
Although hypohidrosis rarely dominates a patient’s dysautonomia, assessment of sudomotor function is often helpful in testing for autonomic impairment (Low, 2004). Widely used tests include the thermoregulatory sweat test (TST), quantitative sudomotor axon reflex test (QSART), and sympathetic skin response (SSR).
Thermoregulatory Sweat Test
The TST is a sensitive semiquantitative test of sweating (Fealey et al., 1989). After a color indicator (quinizarin powder or povidone-iodine) is applied to the skin, the environmental temperature is increased until an adequate core temperature rise is attained (usually a 2°C rise in core temperature or a core temperature of 38.5°C, whichever is less) and the presence of sweating causes a change in the indicator. Thermal stimulation using infrared radiant heat lamps to directly heat the skin are also employed to provide more effective sweat stimulus. Estimating the percent of anterior surface anhidrosis quantitates the results, and the sweat rates may be measured as well. This test has also been helpful in assessing the status of dysautonomias over time. Some characteristic patterns of anhidrosis include (1) the peripheral pattern of distal anhidrosis, seen in distal small-fiber neuropathy and length-dependent axonal neuropathy; (2) the central patterns of distal sparing or segmental involvement, generally seen in MSA or PD; and (3) a sudotomal pattern suggesting involvement at the root or ganglion level, seen in disorders involving nerve roots or specific ganglia, such as diabetes, Sjögren disease, and pure autonomic failure. The TST pattern is therefore helpful in distinguishing between postganglionic, preganglionic, and central lesions.
Quantitative Sudomotor Axon Reflex Test
The physiological basis of the QSART is elicitation of an axon reflex mediated by the postganglionic sympathetic sudomotor axon (Low, 2004). Acetylcholine (ACh) activates the axon terminal. The impulse travels antidromically, reaches a branch-point, then travels orthodromically to release ACh from the nerve terminal. ACh traverses the neuroglandular junction and binds to M3 muscarinic receptors on eccrine sweat glands to evoke the sweat response. The QSART specifically evaluates the functional status of postganglionic sympathetic axons.
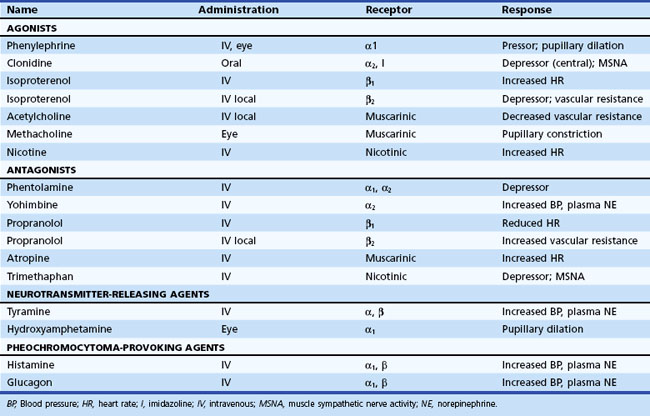
Pharmacological Tests
Information about prevailing sympathetic and parasympathetic activation as well as denervation hypersensitivity can be achieved by use of muscarinic and adrenergic agonists and antagonists (Robertson et al., 2004). Tables 77.4 and 77.5 provide instructive examples of how biochemical and physiological tests may be combined to make novel diagnostic discoveries.
Functional Autonomic Disorders
Functional autonomic disorders are a heterogeneous group of disorders where autonomic nervous system involvement exists, but the role of autonomic dysregulation in the pathogenesis of symptoms is unclear. Included in this group are functional gastrointestinal disorders, interstitial cystitis, migraine, cyclic vomiting syndrome (CVS), fibromyalgia, and chronic fatigue syndrome. Many of these disorders tend to coexist in the same person. For example, about 40% of individuals with migraines have symptoms of IBS and chronic aches and pains that could represent fibromyalgia (Chelimsky et al., 2009). In our experience, children with functional gastrointestinal problems have severe fatigue, headaches, and sleep problems (Ojha et al., 2011). Whitehead et al. (2002) found a high association of functional gastrointestinal disorders with fibromyalgia, chronic fatigue syndrome, temporomandibular joint disorder, and chronic pelvic pain. Often the common denominator is POTS. By report, about 50% of persons with CVS and 40% with migraine report symptoms of orthostatic intolerance (Chelimsky et al., 2009). Sullivan et al. (2005) reported 24 pediatric subjects with functional gastrointestinal syndrome and either POTS, syncope, or both. Interestingly, the gastrointestinal symptoms improved or resolved with treatment aimed at the orthostasis (fludrocortisone, sertraline, and midodrine). Chronic fatigue has been described in association with POTS (Hoad et al., 2008).
Reflex Syncope
Syncope of any type is defined as sudden transitory loss of consciousness with spontaneous recovery that is associated with a loss of postural tone (Mathias et al., 2001). Syncope accounts for more than 1% of hospital admissions. The causes of syncope range from benign to life threatening. The common underlying mechanism of syncope is a transitory decrease in cerebral perfusion. Reflex syncope (fainting) is the most common type of syncope, especially in patients without evidence of structural heart disease (Benditt, 2006; Strickberger et al., 2006). Reflex syncope most commonly occurs while the patient is standing but also occurs while seated and occasionally even while lying during sleep (Jardine et al., 2006). Finally, it can occur with exercise (at initiation or at peak exercise) or with emotional/psychological triggers (e.g., phlebotomy).
Many patients can simply be reassured about the usual benign course of reflex syncope and instructed to avoid those situations that precipitate fainting. The use of support stockings or increased salt intake may help. In young non-hypertensive patients, the most frequently affected, we utilize 2 grams of salt in the morning and 2 grams in the early afternoon. Most salt is excreted by a normal kidney within 3 to 4 hours. Patients should be taught to recognize an impending faint and urged to lie down (or sit down if that is not possible) quickly. This will not be enough for some patients, and other treatment options such as physical countermaneuvers (Wieling et al., 2004) and tilt training (Ector et al., 1998) may be necessary. These are covered in detail in the treatment section of the chapter, as are pharmacological and other interventions.
Syncopal Migraine
A subgroup of patients with syncope respond poorly to the usual management of salt supplementation and tilt training. On further questioning, these patients may consistently experience a headache with migrainous features immediately prior to or after the syncopal spell. A recent review suggests that this entity, sometimes also termed basilar migraine, may be far more common than previously suspected, accounting for about one-third of patients with syncope referred to an autonomic specialist (presumably a more complex group of patients based on the referral bias) (Curfman et al., 2011). The importance of identifying this diagnosis is its prompt response to anti-migrainous medications such as verapamil and topiramate, in this author’s experience. Further identifying features include an increased duration of loss of consciousness (up to 15 minutes in this series), and longer time to full recovery, as might be expected in a migrainous mechanism.
Carotid Sinus Hypersensitivity
Carotid sinus hypersensitivity is defined as an asystole of 3 seconds, a fall in systolic pressure of 50 mm Hg, or both in response to carotid artery massage in a patient with otherwise unexplained dizziness or syncope (Fenton et al., 2000; Mathias et al., 2001). Estimates are that 35 to 100 patients per million per year present with this condition. Although the condition has been ensconced in the medical literature since the era of Soma Weiss (1898-1942), its definition remains controversial, in part because diagnosis is by manual massage of the carotid sinus, with its inherent variability. The test should be performed with the patient supine during continuous ECG and blood pressure monitoring and recording. Longitudinal massage should be performed for 5 seconds over the site of maximal pulsation of the right carotid sinus, located between the superior border of the thyroid cartilage and the angle of the mandible. If no response is elicited, the massage is sometimes repeated on the left side supine and ultimately also on the right and then left sides with upright tilt. Unfortunately, improved practical methods for diagnosis have not emerged. Clinically, a history may exist of syncopal symptoms associated with neck pressure, a tight collar, turning the head, shaving, or swallowing; syncope may also occur spontaneously. Hypotension, bradycardia, or both may dominate the clinical picture. The form in which bradycardia predominates may be improved by demand pacing. Some patients with carotid sinus syncope ultimately require surgical denervation. Occasionally, additional symptoms of headache, dizziness, vertigo, paresthesias, homonymous hemianopsia, and hemiplegia occur in the absence of measured blood pressure or heart rate change, but this may reflect another mechanism such as a migrainous or ischemic process; the older literature terms this phenomenon Weiss-Baker syndrome.
Postural Tachycardia Syndrome
POTS is defined as an increase of at least 30 bpm on standing, associated with symptoms of sympathetic activation (Freeman et al., 2002; Jacob et al., 2000; Low et al., 1997). Orthostatic symptoms include light-headedness, palpitations, tremulousness, visual changes, discomfort or throbbing of the head, poor concentration, tiredness, weakness, and occasionally fainting. Usually, little or no fall in blood pressure occurs on standing (Shibao et al., 2005), and this characteristic should probably be incorporated into the definition. Patients may also have an elevated plasma norepinephrine concentration of 600 pg/mL or more on standing. Standing plasma norepinephrine levels greater than 2000 pg/mL occur, and such patients require careful study to exclude pheochromocytoma. Many POTS patients also have a bluish-red discoloration of skin in the lower extremities on standing. A reduced plasma volume of about 500 mL is often present.
POTS is estimated to affect 250,000 to 500,000 Americans and causes a wide range of disabilities (Benrud-Larson et al., 2002). A 4 : 1 female preponderance exists, typically in the 15- to 45-year age group. Symptom severity is sometimes catamenial. Possible reasons for these cyclical changes include an estrogen-dependent change in plasma volume or a direct estrogen receptor–mediated modulation of vascular reactivity. Other than essential hypertension, POTS is the most common chronic disorder of cardiovascular homeostasis. It is commonly encountered and accounts for frequent referrals to centers specializing in autonomic disorders.
The etiology of POTS is unknown; indeed, the condition has many different names (Box 77.3) and probably many causes. For many years, such patients were considered deconditioned and encouraged to pursue a vigorous exercise regimen. Although such regimens can be quite effective (Fu et al., 2010), it is nonetheless clear that the disorder did not arise from mere deconditioning. The onset of POTS may be abrupt, suddenly disabling a prior marathon runner or Olympic athlete, and often occurs in the wake of a viral infection, pregnancy, or major surgical procedure, encouraging consideration of an autoimmune etiology.
Box 77.3 Postural Tachycardia Syndrome
Alternative Names
Hyperadrenergic orthostatic hypotension
Orthostatic tachycardia syndrome
Postural orthostatic tachycardia syndrome
Hyperadrenergic postural hypotension
Hyperdynamic β-adrenergic state
Sympathicotonic orthostatic hypotension
Mitral valve prolapse syndrome
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree