EEG, Drug Effects, and Central Nervous System Poisoning
Gerhard Bauer
Richard Bauer
Alarge number of patients examined in an electroencephalographic (EEG) laboratory are under treatment with drugs that alter EEG activity. Therefore, it is important to know changes due to the particular drug(s) used, chronic overdosage, the patterns of overt intoxication, and withdrawal effects. The abundant literature of the topic makes a selection inevitable. This chapter discusses EEG findings of drugs most frequently used in modern medicine, provides an overview of toxic encephalopathies of clinical importance, and concentrates on visual EEG reading. Pharmacogenomics of drug actions and toxicities are not addressed in detail (for summary see Ref. 1).
Superimposed fast frequencies (Fig. 43.1) are suggestive for sedating drugs. Otherwise, EEG abnormalities due to substance intake are unspecific. Diffuse slow activities and coma patterns in cases of severe intoxication cannot be distinguished from those seen with other etiologies. This is also true for epileptiform activities and triphasic waves regardless whether they are encountered in intoxicated patients with or without acute symptomatic seizures. With localized EEG abnormalities an additional focal lesion should be excluded (2).
DRUGS ACTING ON THE CENTRAL NERVOUS SYSTEM
General Anesthetics
Molecular and cellular mechanisms of general anesthesia have been reviewed by Franks and Lieb (3) and Campagna et al. (4). Anesthetics exert their effects primarily at the neuronal ion channels via neurotransmitter receptors and by binding directly to the protein site. Initially, anesthetics induce amnesia, euphoria, analgesia, excitation, and hyperreflexia. Surgical anesthesia consists of deep sedation, muscle relaxation, and diminished or abolished motor and autonomous responses to noxious stimuli. Specific effects of different substances (nitrous oxide, halothane, enflurane, isoflurane, sevoflurane, desflurane, propofol, etc.) are due to specific binding sites and genetic peculiarities of the patient.
Correlation studies of EEG and the stage of anesthesia have been summarized by Winters (5) and Sloan (6). The initial phase is dominated by the appearance of frontal fast activity, gradually becoming more generalized and associated with dissolution of the alpha rhythm. During excitation, epileptiform activities become discernible in nonepileptic and epileptic patients with different anesthetic drugs (for summary see Ref. 7). As anesthesia deepens, activity becomes slower and the voltage increases. Eventually, a burst-suppression pattern can be observed. At the deepest level, EEG activity ceases. The burst-suppression pattern exhibits no differences to those seen with deep comatose states under pathologic circumstances (see Chapter 23) or intoxication with sedative drugs (see below).
HYPNOTICS AND SEDATIVES
Barbiturates
Barbiturates remained on the market only as anesthetics and antiepileptic drugs, especially in developing countries (8). They apparently act at all levels of the neuraxis by binding to a specific site on the γ-aminobutyric acid (GABAA) receptor, different from the binding site of benzodiazepines (9). Low blood concentrations produce inhibition of higher cortical functions and disinhibition of more primitive behavior. With increasing blood levels, clinical signs are determined by generalized inhibition.
EEG Changes with Therapeutic Doses
When administered in small doses, the barbiturates produce an increase in fast activities in the range of 25 to 35 cycles/sec (Hz), soon shifting to 15 to 25 Hz. This activity is predominant over the frontal cortex and spreads to parietal and occipital areas. Increasing doses are accompanied by intermixed slow activities and dissolution of the alpha rhythm, indicating drowsiness and sleep. In children, the elderly, and patients with organic brain lesions, barbiturates even in low doses may induce irritability, hyperactivity, or delirium (10). Lack of barbiturate-induced fast activity indicates a diffuse organic brain lesion; asymmetries are suggestive of a focal lesion (11).
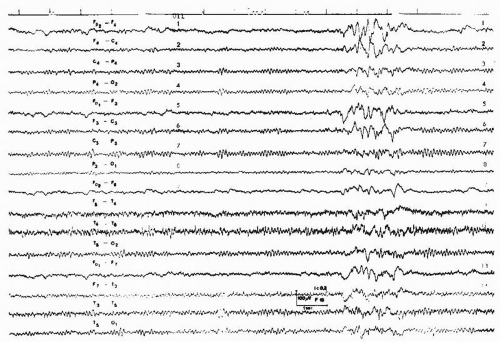
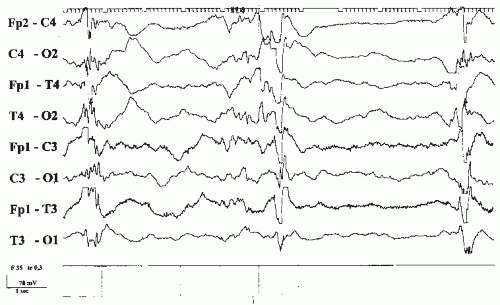
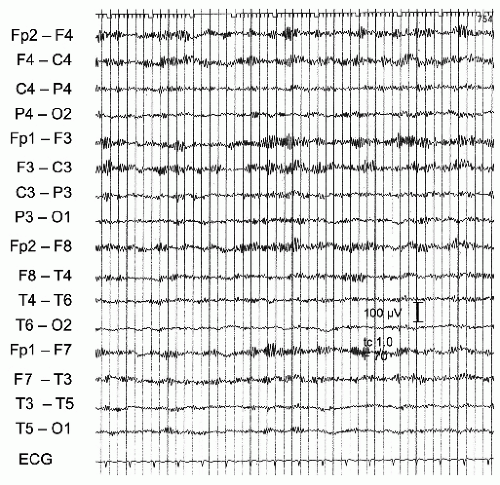 Figure 43.1 St.St., a 14-year-old female teenager, who took an unknown amount of diazepam. Clinically slight drowsiness. Twelve- to 13-Hz rhythmical activities, maximal over the anterior regions. |
Chronic Overdosage
Signs of chronic barbiturate intoxication in seizure patients might be misinterpreted as personality changes attributed to the epileptic disorder. Signs include general sluggishness, lethargy, difficulty in thinking, poor memory, learning disabilities, and cerebellar deficits (10). Chronic neurotoxicity may result in insidiously developing disturbances of higher cognitive functions without concomitant abnormalities (12). Compared with other antiepileptic drugs, cognitive adverse effects are pronounced with phenobarbital (for review see Ref. 13). EEG changes are similar to those seen in the first stage of acute intoxication. In epileptic patients, an increase in slow activities in serial recordings without increase or even with reduction in seizure frequency is indicative of overdosage and should prompt an evaluation of the serum level.
EEG Changes after Withdrawal
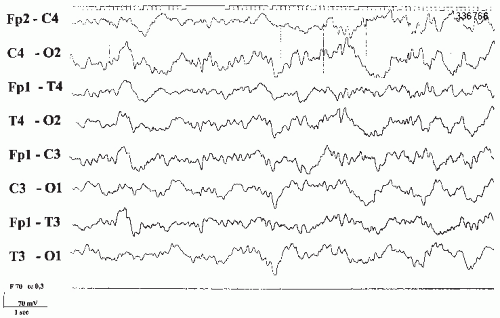
After long-term ingestion of barbiturates, abrupt withdrawal leads to paroxysmal abnormalities. Myoclonic jerks, even as myoclonic status, generalized tonic-clonic seizures, and delirium are major complications. The EEG frequently shows generalized paroxysmal activities and spikes, especially with photic stimulation (14). These changes are usually transient in nature and most often occur within the first few days after withdrawal, but they may occasionally persist for 3 to 4 weeks. They also occur without clinical seizures and even with medically controlled gradual withdrawal (Fig. 43.2).
Acute Intoxication
Sedatives such as barbiturates, anxiolytic drugs, phenothiazines, and tricyclic antidepressants can all produce coma. They directly depress cellular oxidative metabolism and do not permanently damage neuronal functions. The mere functional character of CNS depression after an acute overdose indicates a less grave prognosis even in the presence of very serious clinical and EEG signs.
According to the multilevel action of barbiturates, the clinical syndrome encompasses cortical, reticular, vestibular, and other brainstem dysfunctions. Coma due to overdose with sedatives is fairly characteristic if one considers the depth of
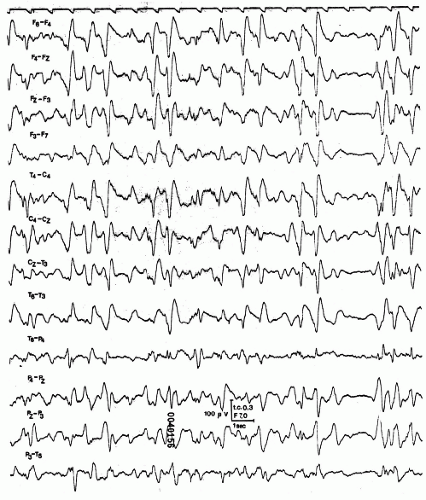
unresponsiveness in combination with flaccid muscle tone, absent plantar responses, and preserved pupillary reactions (15). With fast-acting barbiturates cerebral functions can be depressed in a rostro-caudal fashion and flexor and extensor postures may initially evolve (Fig. 43.3) (16). In severe cases, circulatory effects lead to a typical shock syndrome. Subsequent cerebral hypoxia may turn the functional disturbance into structural damage with serious prognostic implications.
unresponsiveness in combination with flaccid muscle tone, absent plantar responses, and preserved pupillary reactions (15). With fast-acting barbiturates cerebral functions can be depressed in a rostro-caudal fashion and flexor and extensor postures may initially evolve (Fig. 43.3) (16). In severe cases, circulatory effects lead to a typical shock syndrome. Subsequent cerebral hypoxia may turn the functional disturbance into structural damage with serious prognostic implications.
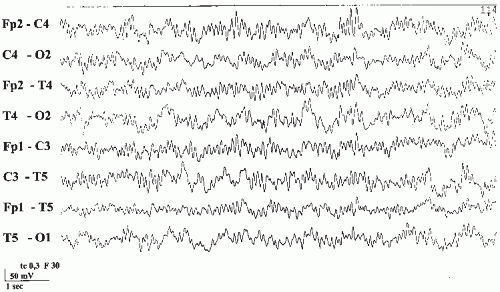 Figure 43.3 E.A., a 24-year-old female. Suicidal attempt by ingestion of a combination of cyclobarbital, hexobarbital, meprobamate, and carbromal (= Somnupan, banned from the Austrian market for several years). Coma with decerebrate posturing (with fast-acting barbiturates cerebral functions can be depressed in a rostro-caudal fashion and motor signs may initially evolve) (16). EEG with diffuse slow activity superimposed by high-voltage 10- to 13-Hz activities. Patient recovered completely. |
EEG signs in different stages of acute intoxication closely resemble those observed with other CNS depressant substances. Initial dissolution of the alpha rhythm and appearance of interspersed theta frequencies are followed by predominant slow activities superimposed by 10- to 16-Hz rhythmical activity maximal over the anterior regions. Such an EEG is suggestive of intoxication with depressant drugs of any type. However, a so-called spindle coma after head injury and the alpha coma pattern with cerebral hypoxia may look similar. Superimposed fast activities after intoxications have also been termed “drug-induced alpha coma” (17). The separation from alpha coma pattern with hypoxic states has prognostic significance with a much more favorable outlook with intoxications (17). With deepening coma, fast frequencies disappear and diffuse delta activity becomes prominent. With impending breakdown of vegetative functions, periods of flattening and, eventually, a burst-suppression pattern appears (18). Preceding or following the development of a burst-suppression pattern, the record may be characterized by bi- or triphasic sharp transients (Fig. 43.4) (19). Electrocerebral silence signifies the most advanced cases. The prognostic meaning of this otherwise ominous sign is less grave with intoxication. Several patients survived without permanent neurologic sequels (20).
Miscellaneous Hypnotic Drugs
Melatonin
Melatonin has been extensively discussed in the popular media. It is the hormone of darkness (22). The substance may act as a phase-setter for sleep-wake cycles in subjects with a delayed sleep phase syndrome (23). However, no evidence was found that melatonin is effective in treating secondary sleep disorders or jet lag and shift work disorders (24). For difficulties in sleep onset, ramelteon, a melatonin receptor agonist was recommended (25). Effects on the conventional EEG have not been reported for melatonin or the agonist.
Bromides
Bromide was the drug of choice as anticonvulsant and sedative during the second half of the 19th century. It is still used in the treatment of therapy-resistant tonic-clonic seizures (26). With acute bromide intoxication, the EEG shows mixed slow and fast activities. Very pronounced EEG slowing is found in chronic bromide encephalopathies. Drug level determinations have improved the handling of the drug, so the typical bromism of the old days has disappeared.
Antipsychotic Drugs
Antipsychotic drugs are used for the treatment of schizophrenia. They are listed as typical, classic or first-generation antipsychotic agents (chlorpromazine, chlorprothixen, flupentixol, melperone, fluphenazine, perphenazine, trifluoperazine, thiothixene, haloperidol among 18 drugs developed between the 1950s and the 1970s), and atypical or second-generation drugs (clozapine, risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, amisulpride). Major progress has been made to elucidate their mechanism of action (for review see Ref. 27). Typical antipsychotic drugs act by blocking the dopamine D2 receptor and are connected with parkinsonian side effects. The newer antipsychotic compounds bind at many different receptors including members of the dopamine receptor family and 5-HT2A receptors.
Typical Antipsychotic Drugs
EEG changes due to neuroleptics are not particularly different for the numerous substances under this pharmacologic heading and therefore are discussed jointly.
EEG Changes with Therapeutic Doses
A great number of quantitative analyses of EEG changes due to therapeutic doses of neuroleptics have been summarized by Saletu (28). Neuroleptics increase the alpha activity with a slight shift to the lower frequency range and increase the amount of slow activity and the general voltage output, but decrease percent time of beta activity, variability of frequencies, and the average frequency. Mild to moderate diffuse abnormalities have been observed on visual EEG reading with considerable but inconsistent variations among the different substances (29). The EEG abnormalities in treated patients exceeded the occurrence in psychiatric patients not treated with antipsychotics for at least 5 days.
Epileptogenic Potency
Although the risk of seizures under treatment with typical antipsychotic drugs does not differ substantially from the general population (30), with high doses seizures and spikes in the EEG may occur (31). EEG and clinical seizures can deteriorate in chronic epileptic patients. Nonconvulsive status epilepticus has been reported under treatment with typical neuroleptics (32).
Acute Intoxication
Overdosage with neuroleptics usually leads to sedation and coma. In milder cases, patients can be agitated, delirious, or confused. Involuntary extrapyramidal movements, parkinsonism, and generalized tonic-clonic seizures can be prominent. Fatalities are rare if neuroleptics alone are taken; death is attributable to cardiac effects. The EEG is dominated by slow waves, frequently occurring as generalized paroxysmal activities. Fast frequencies as in barbiturate poisoning are not recorded (14).
Atypical Antipsychotic Drugs
A long list of newer antipsychotic drugs is on the market (clozapine, amisulpride, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, zotepine, aripiprazole). In a systematic meta-analysis only clozapine exhibited fewer parkinsonian symptoms and a higher antipsychotic effect than conventional drugs (33). Compared with the classic antipsychotics, the newer drugs are less extensively studied with quantitative EEG methods.
Clozapine
Clozapine has affinity for serotonin (5-HT2a, 5-HT6, 5-HT7, 5-HT2c, and 5-HT3), alpha-adrenergic, and dopamine D4 receptors but weak affinity for the D2 receptor (34). It causes no extrapyramidal symptoms and tardive dyskinesia (33). The major adverse effect of clozapine relates to its potential for damaging the granulocyte cell line. A cumulative risk figure for agranulocytosis of 0.8% at 1 year and 0.9% at 18 months was calculated (35).
EEG power spectra show an increase in delta, theta, and above-21-Hz beta activities. The EEG became abnormal in up to 53% of patients as a function of clozapine serum levels (36). Increased theta and delta activities were prominent over the frontal, central, and parietal areas (37). EEG abnormalities included paroxysmal slowing and spikes. Clozapine can provoke seizures. Seizure frequency amounts up to 4% (38), increases with doses over 600 mg/day, and seems to be greater than with other typical and atypical antipsychotic drugs (39). Besides generalized tonic-clonic seizures, myoclonic jerks have been observed (40).
Risperidone
Risperidone is a benzisoxazole derivative with combined dopamine D2 receptor and serotonin 5-HT2 receptor blocking properties (41). The substance has an established efficacy in acute psychotic states. Risperidone does not induce EEG changes in the waking state (42). The risk of risperidone-related seizures amounts to 0.3%, a low number compared with the 3.5% risk of clozapine, that ranks highest among all antipsychotic drugs (39).
Other Atypical Antipsychotic Drugs
Little is known about EEG changes of other new antipsychotic drugs. After Alper et al. (39) the seizure risk extracted from approval reports is 0.9% for olanzapine, 0.8% for quetiapine, 0.4% to 0.5% for ziprasidone, and 0.4% for aripiprazole.
Antidepressant Drugs
Since the introduction of the first-generation tricyclic antidepressants, several new drugs have been marketed. New
antidepressants might be classified according to their central mode of action into selective serotonin reuptake inhibitors (fluoxetine, fluvoxamine, paroxetine, citalopram, sertraline), dual serotoninergic antidepressants (reuptake inhibitors plus receptor antagonism; nefazodone), selective serotonin and noradrenalin reuptake inhibitors (venlafaxine), noradrenergic and specific serotonergic antidepressants (mirtazapine), selective noradrenalin reuptake inhibitors (reboxetine), and reversible specific monoamine oxidase inhibitors (moclobemide) (43).
antidepressants might be classified according to their central mode of action into selective serotonin reuptake inhibitors (fluoxetine, fluvoxamine, paroxetine, citalopram, sertraline), dual serotoninergic antidepressants (reuptake inhibitors plus receptor antagonism; nefazodone), selective serotonin and noradrenalin reuptake inhibitors (venlafaxine), noradrenergic and specific serotonergic antidepressants (mirtazapine), selective noradrenalin reuptake inhibitors (reboxetine), and reversible specific monoamine oxidase inhibitors (moclobemide) (43).
Antidepressants, especially amitriptyline, and several antiepileptic drugs (see below) are also important in the treatment of neuropathic pain (44).
Tricyclic Antidepressants
EEG Changes with Therapeutic Doses
Tricyclic antidepressants such as imipramine, amitriptyline, doxepin, desipramine, nortriptyline, and protriptyline increase the amount of slow and fast activities along with instability of frequencies and voltage. Furthermore, they slow down the frequency of the alpha rhythm (for review see Ref. 28).
Epileptogenic Potency
Paroxysmal slow waves, spikes, and polyspikes occur with therapeutic doses (45,46). The seizure frequency may increase in chronic epileptic patients. Furthermore, single or multiple seizures occur in nonepileptic patients, especially with high doses (47,48). Several cases of absence status have been thought to be due to treatment with tricyclic agents (49,50).
Acute Intoxication
Unlike the phenothiazines, tricyclic antidepressants have much narrower therapeutic ranges and quickly reach toxic levels. Overdosage may result in serious life-threatening conditions. This is of great concern, because depression is notorious for suicidal attempts. The clinical picture is characterized by hyperpyrexia, hypertension, seizures, and coma. The prognosis depends largely on the effects on the cardiovascular system. Even with therapeutic doses, there is an increased tendency toward cardiac arrhythmias, and there have been several reports of unexpected death. Greater than 10-fold differences in the number of deaths per million prescriptions have been shown comparing tricyclic antidepressants with the newer antidepressants (51). The EEG during acute intoxication shows widespread, poorly reactive, irregular 8- to 10-Hz activity and paroxysmal abnormalities including spikes as well as unspecific coma patterns (Fig. 43.5) (14).
New Antidepressants
Seizures have also been reported with the newer antidepressants. There are differences in seizure propensity among the different substances, particularly pronounced with maprotiline and bupropion (1.5% seizure risk) and low with trazodone, nefazodone, mirtazapine, and the selective serotonin reuptake inhibitors (for review see Refs. 39, 43, and 52). However, second-generation antidepressants other than bupropion apparently have antiepileptic properties (39).
In general, the new antidepressant drugs have a higher therapeutic index than the tricyclic compounds. Overdosage with several of the new drugs has been reported, but deaths seem to be an exception (for review see Ref. 43). Fatal toxicity index for venlafaxine is higher than that for other serotoninergic antidepressants (51). EEG signs during the intoxicated state are rarely demonstrated and show no particular features.
The Serotonin Syndrome
The serotonin syndrome is an adverse drug reaction associated with a number of drug-drug interactions (53). Among the drugs at risk are selective serotonin reuptake inhibitors, other antidepressant drugs, and monoamine oxidase inhibitors. The protean manifestations of the syndrome include mental status changes, autonomic hyperactivity, and neuromuscular abnormalities.
Since the condition is potentially life-threatening, an early diagnosis with immediate removal of the responsible drugs is essential. Case reports of the syndrome have noted EEG abnormalities with diffuse slow activities, spikes, and triphasic waves (54).
Since the condition is potentially life-threatening, an early diagnosis with immediate removal of the responsible drugs is essential. Case reports of the syndrome have noted EEG abnormalities with diffuse slow activities, spikes, and triphasic waves (54).
Lithium
Lithium is still used in the prophylactic treatment of bipolar mood disorders and has not been entirely replaced by valproate or lamotrigine. EEG changes during the treatment are frequent and marked (55,56). In general, they parallel the blood serum levels, but there are also remarkable discrepancies.
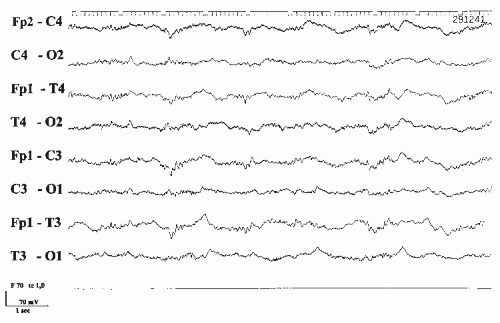
Acute intoxication with lithium salts can be a life-threatening condition. Early symptoms include fatigue, muscular weakness, and tremor. When plasma concentrations rise above 2 mEq/L, more serious toxic effects occur. Disturbances in renal elimination or fever with liberation of tissue-bound lithium are the most frequent causes of intoxication. Obtundation, stupor, or delirium is always present. Neurologic signs show a bewildering variety of cortical and subcortical dysfunctions, optomotor disturbances, and peripheral neuropathies (57,58). Movement disorders are especially dramatic. Myoclonic jerks (59), convulsions, choreiform hyperkinesis, other complex extrapyramidal movements, and several other signs may be observed. If accompanied by triphasic waves in the EEG, such conditions have been called lithium-induced Creutzfeldt-Jakob syndrome (60) or nonconvulsive status epilepticus (61). However, the epileptic or encephalopathic nature of these confusional states remains undecided (62). Permanent neurologic sequels due to lithium toxicity have also been reported (63).
The EEG is always markedly abnormal with lithium intoxication and shows diffuse slowing, paroxysmal abnormalities including spikes and triphasic waves. Focal slowing also occurs and does not have to be taken as a sign of focal brain lesion (64). Improvement of electrical activity parallels clinical improvement, but the EEG abnormalities regularly outlast the abnormal serum levels (Fig. 43.6A and B).
ANXIOLYTIC DRUGS
Benzodiazepines
The main actions of benzodiazepines can be described as hypnotic, anxiolytic, anticonvulsant, myorelaxant, and amnesic. They exert their actions by binding at specific sites at the GABA-A receptor chloride ionophore (65). The distribution of GABA-A receptors within brain regions and changing compositions of receptor subunits might contribute to differences in the efficacy of the numerous benzodiazepines (9,66). Furthermore, pharmacokinetic properties like rapidity of absorption, half-life time, binding to fat deposits, and activities of metabolites are important differences among the substances. In general, however, all benzodiazepines exert actions mentioned above and share the same side effects. Therefore, those on the list of benzodiazepines (clobazam, clonazepam, diazepam, estazolam, flunitrazepam, flurazepam, lorazepam, nitrazepam, quazepam, temazepam, triazolam among others) are considered together.
EEG Changes with Therapeutic Doses
The benzodiazepine derivatives are potent activators of beta activity, which persist in the EEG as long as 2 weeks after the last ingestion. As with barbiturates, benzodiazepine-induced fast activities are reduced over the site of a cerebral lesion (11). Furthermore, benzodiazepines produce a decrease in alpha activity and general voltage, and a slight increase of 4- to 7-Hz activity (28).
Effect on Seizures
Benzodiazepines reduce generalized spikes but have no effect on interictal focal spikes (67) except for discharges in rolandic
epilepsy (68). They are drugs of first choice for status epilepticus and repetitive acute symptomatic seizures (69). Despite their antiepileptic action, they may provoke tonic status epilepticus if intravenously administered to children with absence status in Lennox-Gastaut syndrome (70,71). Lorazepam is superior in the treatment of status epilepticus because it is less extensively bound to fat (72). Withdrawal of benzodiazepines has been considered an etiologic factor of “de novo” absence status of late onset (50).
epilepsy (68). They are drugs of first choice for status epilepticus and repetitive acute symptomatic seizures (69). Despite their antiepileptic action, they may provoke tonic status epilepticus if intravenously administered to children with absence status in Lennox-Gastaut syndrome (70,71). Lorazepam is superior in the treatment of status epilepticus because it is less extensively bound to fat (72). Withdrawal of benzodiazepines has been considered an etiologic factor of “de novo” absence status of late onset (50).
Side Effects of Benzodiazepines
All benzodiazepines share a long list of side effects consisting of tiredness during the day following ingestion (hangover), rebound anxiety, anterograde amnesia, rebound insomnia, low-dose dependency, and withdrawal syndromes. The profile of side effects depends on the specific binding site, the dose, the half-life time, and other pharmacokinetic variables. With shortacting substances, the amnesic effects are comparatively marked (73,74). In general, benzodiazepines have significantly fewer side effects than barbiturates, and it is rational to prefer them as hypnotics.
Acute Intoxication
The clinical picture resembles that seen with other CNS depressant drugs and is not truly specific for benzodiazepines (75). The prognosis is generally good, although in patients with decreased respiratory reserve and in the very young even therapeutic doses may dangerously depress cardiorespiratory function (76). The EEG shows prominent fast activity with no response to stimuli. With larger doses, coma patterns, as in other intoxications, are recorded (14).
So-called Z-drugs
Zaleplon, zolpidem, and zopiclone are non-benzodiazepine hypnotics. Zolpidem belongs to the class of imidazopyridines (77). It binds to the alpha 1 unit of the GABA-A receptor complex (9). Similar side effects as with benzodiazepines have been reported. Sleep architecture is claimed to resemble normal patterns more closely. Zopiclone belongs to the cyclopyrrolones. The final pathway constitutes an opening of the chloride ionophore. The most frequent adverse effects include bitter taste, dry mouth, and complaints comparable to the benzodiazepines (78). Microstructural analysis of sleep architecture and decrease of EEG arousals allow discrimination of benzodiazepines, zolpidem, and zopiclone (79). There was no consistent pattern of superiority in therapeutic efficacy among the Z-drugs.
Baclofen
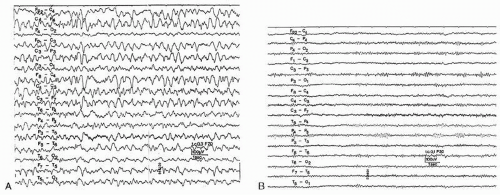
Baclofen is a selective GABA-B receptor agonist and is used for treatment of spasticity (80). Oral and intrathecal baclofen have been associated with epileptic seizures (Fig. 43.7) (81,82). After intrathecal baclofen overdose the EEG can exhibit coma patterns characterized by periodic generalized epileptiform discharges without overt seizures (83).
Psychotogenic Drugs
The substances under the heading of psychotogenic drugs are intimately connected with addiction. Exciting progress has been made elucidating the neurobiology of addiction and its relation to various intrinsic neurotransmitter systems (84). This scientifically as well as clinically important issue can only be touched on a few references.
Lysergic Acid Diethylamide (LSD) and Mescaline
Marijuana
Cannabis exerts its central effects through the CB1 cannabinoid receptor (for review see Ref. 86). These effects include disruption of psychomotor behavior, short-term memory impairment, stimulation of appetite, and antinoceptive and antiemetic
actions. Neuropsychologic deficits have been noted after years of heavy frequent cannabis use (87), and the risk of psychosis in later life seems to be increased (88). Cannabis is used in the treatment of neuropathic pain and spasticity (89), but the risk to benefit ratio has to be clarified.
actions. Neuropsychologic deficits have been noted after years of heavy frequent cannabis use (87), and the risk of psychosis in later life seems to be increased (88). Cannabis is used in the treatment of neuropathic pain and spasticity (89), but the risk to benefit ratio has to be clarified.
CNS Stimulants
Central stimulants potentiate central dopamine activity. Different modes of dopaminergic potentiation have been shown in such drugs as the amphetamines, methylphenidate, and cocaine (92).
Low-Dose Effects on EEG
CNS stimulants increase the amounts of beta and alpha activities, reduce the general voltage and the amount of slow waves, and tend to suppress seizure discharges in pertinent cases, especially the 3-Hz spike-and-wave complexes (28). Abnormal slow waves during stupor and coma are diminished by the administration of methylphenidate. So-called recreational club drugs illegally offered as “ecstasy” contain CNS stimulants, especially methamphetamines and dioxymethamphetamine (MDMA) (93). A number of acute and long-lasting medical complications are associated with abuse of these drugs. Therefore, most CNS stimulants have been banned from the market. Modafinil, a pharmacologically unique wake-promoting agent, has replaced the traditional amphetamines for the treatment of narcolepsy (94). Psychostimulants are also used for treatment of attention-deficit hyperactivity disorder (ADHD). Increased slow activities in unmedicated ADHD girls are normalized under psychostimulant therapy (95). With atomoxetine a nonstimulant pharmacotherapy is now available for ADHD (96).
Acute Intoxication
Intoxications with CNS stimulants have increased due to their illegal use as “ecstasy.” In a 1996 survey it was reported that nearly 5 million Americans have used methamphetamine at some time in their lives (97). Symptoms of mild intoxications are those of sympathetic activity. In more severe poisoning, hypertension, confusion, cardiac anomalies, and, finally, hyperthermia, convulsions, circulatory failure, and coma occur. Death due to amphetamines is related either to direct pharmacologic effects or to secondary complications in drug addicts (98). The EEG with overdose of stimulant drugs shows the usual coma pattern without any particular features. Methamphetamines can damage dopaminergic and serotoninergic neurons (99) and produce corresponding permanent neurologic abnormalities.
Cocaine
Cocaine has become a major substance in the field of drug addiction. It binds strongly to the dopamine reuptake transporter and blocks such reuptake after normal neuronal activity. High dopamine concentrations at the synapse produce the characteristic cocaine “high” (100). The induction of long-term synaptic plasticity in neurons of the brain’s reward system seems to represent a common pathway for the addictive potential of cocaine, morphine, nicotine, ethanol, and amphetamine (101). Variants of the DRD2 gene have been associated with cocaine, nicotine, and opioid dependency (102).
Berger’s historic experiments on the EEG effects of cocaine intake have been confirmed. Cocaine increases beta power correlated with the area under the cocaine plasma versus time curve (103). Several neurologic complications are associated with chronic cocaine intake. It induces strokes (104, 105, 106 and 107), orbital infarction (108), subarachnoid and intracerebral hemorrhages (109,110), vasospasms after aneurysmal subarachnoid hemorrhage (111), cerebral vasculitis (112), persistent dyskinesias (113), oculomotor nerve palsies (114), and spinal and medullary vascular syndromes (106), and can provoke seizures or exacerbate a preexisting seizure disorder (92). Seizure activity can present as status epilepticus (115).
Antiepileptic Drugs
Drug therapy of epilepsies represents the long-term therapy par excellence. Dose- or interaction-related CNS toxic effects are common. Delayed toxic effects and drug-induced diseases are not dose-related, occasionally life-threatening and not always reversible. Antiepileptic drugs act directly on voltage-gated ion channels and by influencing GABA-mediated effects. They can bind at the GABA-A receptor at different binding sites, or can inactivate GABA-metabolizing enzymes and inhibit uptake of GABA into nerve cells and glia cells. Other antiepileptic drugs influence different transporter systems or the glutaminergic system via NMDA/AMPA receptors. For detailed and comprehensive information, the reader is referred to Levy et al. (116).
Several antiepileptic drugs are also used in the treatment of neuropathic pain, migraine, and alcohol dependency, and as so-called mood stabilizers.
EEG changes due to antiepileptic drugs have to be divided into effects of therapeutic doses on background activity and on frequency and morphology of preexisting spikes as well as into the effects of overdoses, overt intoxication, and withdrawal. In general, the standard antiepileptic drugs slow down the frequency of the occipital basic rhythm even with nontoxic serum levels and increase the percentage of power in the theta and delta bands with visual (117,118) and with quantitative analysis (119). These changes correlate with cognitive effects and subjective complaints. The effect on interictal spikes varies considerably with a positive correlation between seizure frequency and the number of spikes in some patients (120).
Hydantoins
Signs of cerebellovestibular dysfunction signal initial hydantoin overdosage. Cerebellar atrophy occurs with chronic hydantoin use but also with acute intoxication (121,122). Overt hydantoin intoxication is further characterized by altered higher cognitive function, pyramidal signs, and several extrapyramidal movement disorders. Epileptic seizures may be exacerbated (123,124). Cardiovascular toxicity is rare unless the substance has been given parenterally for treatment of status epilepticus.
In contrast to barbiturates and benzodiazepines, the EEG shows no increase in fast activities with visual EEG reading (125). Phenytoin increases the power in the theta and delta bands with blood levels in the usual range and without clinical signs of overt intoxication (117,118). There are conflicting reports on the influence of phenytoin on interictal epileptiform discharges. No changes (126), an increase (127), or a decrease of spikes (128) have been reported.
No changes in background activity occurred with reduction of phenytoin dosage (Duncan et al., 1989). Withdrawal of antiepileptic drugs routinely is used as a seizure-provoking method in intensive epilepsy monitoring. No misleading information was gained with this procedure localizing the seizure onset zone (129). The same was true withdrawing carbamazepine and valproate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree