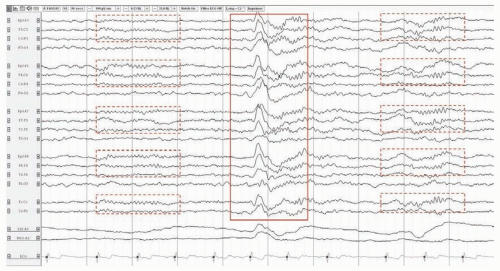
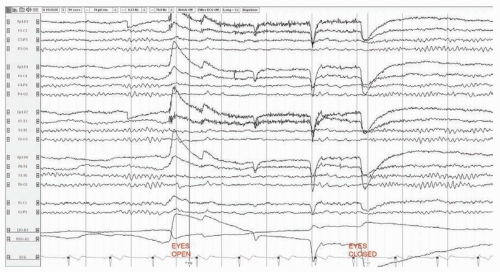
 FIGURE 25.1 Normal EEG in an awake 37-year-old man. The posterior dominant rhythm attenuates with eye opening. |
most children at 4 years. Sleep spindles appear at 2 months but do not become synchronous in most children until the age of 2 years. Normal delta activity persists during wakefulness through childhood, adolescence, and early adulthood but becomes progressively less prominent and more intermittent and restricted to the posterior regions (posterior slow wave of youth).
can occasionally be seen in the aftermath of a seizure in patients with chronic epilepsy or with a remote brain injury. Rarely, LPDs are associated with clinical manifestations (time-locked contralateral myoclonus, visual hallucinations, etc.).
TABLE 25.1 Electroencephalography Features of Major Epileptic Syndromes | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
A suppression-burst pattern in which periods of suppression alternate with bursts of high amplitude activity, can also be seen in severe cases (Fig. 25.6). Generalized rhythmic delta activity (GRDA; often frontal and intermittent; FIRDA) is another nonspecific finding of encephalopathy, although it can be seen with lesions in the frontal lobes. Generalized periodic discharges (GPDs) are repetitive waveforms that occur either at periodic or quasiperiodic intervals of 0.5 to 3 cps. They can be encountered in any type of encephalopathy, at a moderate to severe stage, and are associated with an increased risk of seizures. Triphasic waves are a subtype of GPDs with a typical morphology (Fig. 25.7). They were initially thought to be specific of hepatic encephalopathy. They can in fact be seen in most metabolic or toxic encephalopathies and even during or after status epilepticus. A major role of the EEG in patients with acute confusion and obtundation is to identify those who suffer from nonconvulsive status epilepticus.
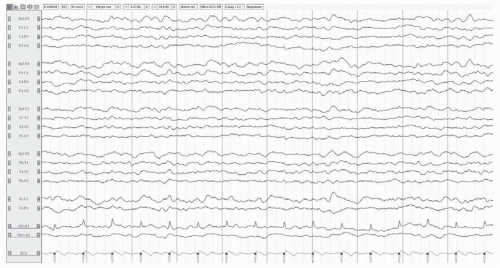 FIGURE 25.3 Diffuse slowing in a 72-year-old woman with sepsis. The posterior dominant rhythm is not visible. The background consists of admixed theta and delta activity.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|