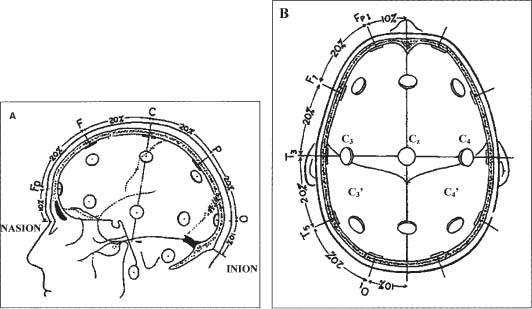
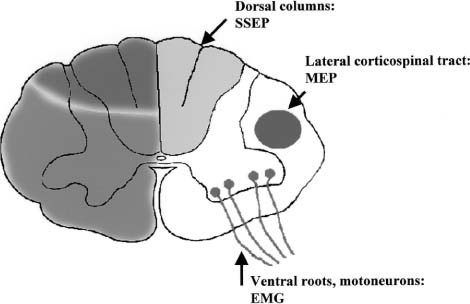
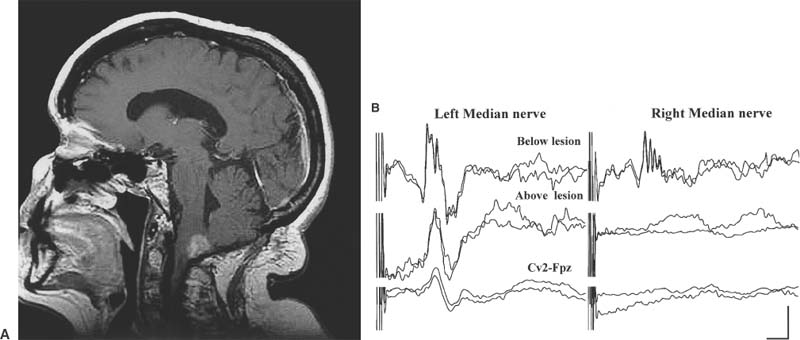
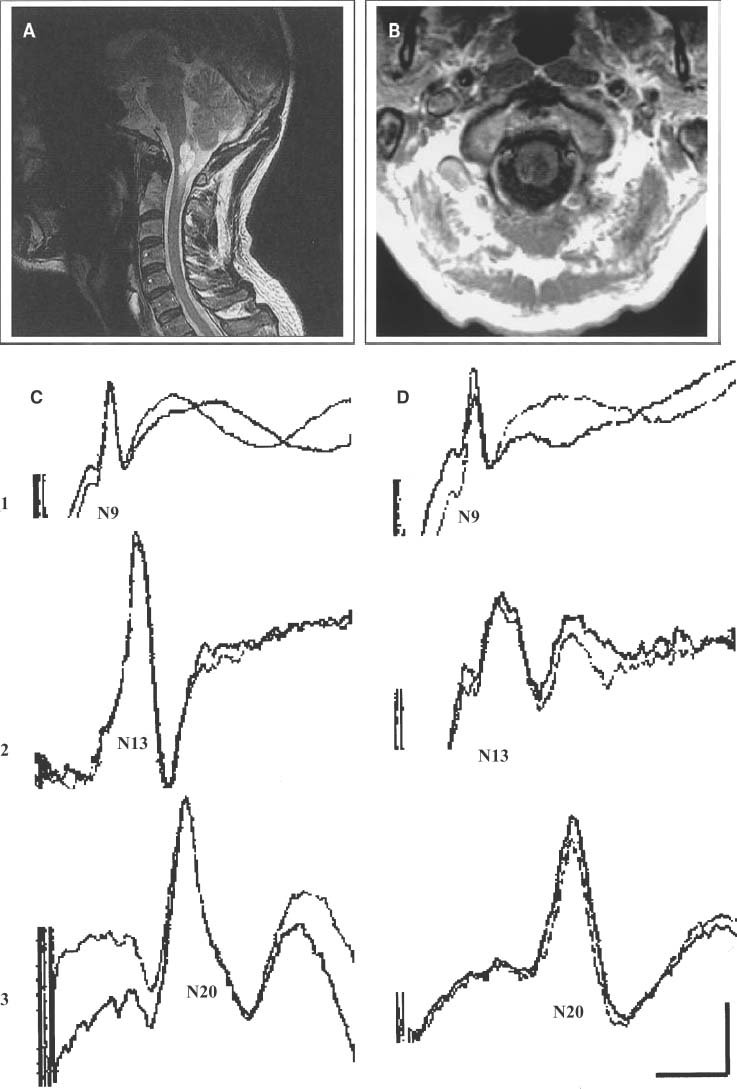
15 With advances in magnetic resonance imaging (MRI) techniques, neurosurgeons presently are provided with precise diagnostic imaging of spinal cord tumors. Consequently, patients with spinal cord tumors are more likely to be diagnosed accurately and will undergo surgical treatment. One of the major issues that the neurosurgeon has to face in the resection of tumors of the spinal cord or peripheral nerves is that the tumor may compress and distort the complex neural tissue, which will make it extremely difficult to recognize normal and physiologically intact structures. Thus, intraoperative electrophysiologic monitoring could be very useful to minimize iatrogenic neurologic injury and to assess the physiologic integrity of the spinal cord and peripheral nerves during surgical treatment of spinal tumors. At least three major functions of intraoperative electrophysiologic monitoring during spinal surgical intervention are recognized. First, intraoperative monitoring is crucial to directly monitor the neurologic function of those structures that are at risk during the surgery. Second, intraoperative monitoring is a valuable diagnostic tool to identify and map eloquent neural structures during tumor resections. Finally, intraoperative electrophysiologic monitoring may provide additional data to predict the neurologic outcome of neurosurgical intervention during tumor resection. Increasing clinical data suggest that the detection of electrophysiologic changes will alert the surgeon and allow for timely intervention, resulting in preservation or even improvement of neurologic outcomes.1,2 This chapter introduces the full spectrum of the presently available intraoperative monitoring modalities and the current state of the art on the use of these methods during neurosurgery for the spine, spinal cord, or peripheral nerve tumors. It is important to remember that mechanical or vascular trauma may selectively affect different strictures and tracts within the spinal cord. With regard to electrophysiologic intraoperative monitoring, two major spinal tracts will be considered: the dorsal ascending columns and the descending lateral corticospinal tracts. A schematic diagram of the localization of the monitorable spinal pathways and spinal cord blood supply is presented in Fig. 15-1. The dorsal columns of the spinal cord with the fasciculus gracilis (lower extremity) and fasciculus cuneatus (upper extremities) convey proprioceptive information as well as vibration sense from the joints and ligaments. The somatosensory evoked potentials (SSEP) and spinal cord evoked potentials (SCEP) are used intraoperatively to monitor this portion of the spinal cord.3–6 The lateral corticospinal tracts are localized within the lateral aspects of the spinal cord and convey the major descending motor signals to activate the spinal cord motor neurons. Motor-evoked potentials (MEP) have been utilized as a means to intraoperatively monitor these important ventrally localized pathways of the spinal cord.2,7,8 A novel sensory-evoked potential method for selective monitoring of the ventral portion of the spinal cord-was proposed and evaluated in a neurosurgical setting: cerebellar-evoked potentials (CEPs).9 Finally, the integrity of the motor neurons within the ventral horn and ventral spinal roots can be monitored intraoperatively using electromyography (EMG).10–12 FIGURE 15-1 Schematic diagram of the cross section of the spinal cord. The blood supply of the white and the gray matter of the spinal cord is indicated on the left portion of the diagram. Posterior spinal arteries: blood supply to the posterior third of the spinal cord (blue-shaded area). Anterior spinal artery: blood supply to the anterior two thirds of the spinal cord (green-shaded area). The major descending and ascending pathways that could be monitored intraoperatively are indicated on the right portion of the diagram. SCEP, spinal cord-evoked potentials; SSEP, somatosensory-evoked potentials; MEP, motor-evoked potentials; EMG, electromyography. Advances in neuroimaging, such as MRI, have significantly improved our ability to diagnose intramedullary lesions; however, they have not solved the problem of functionally assessing involved neural structures. Moreover, in some instances the extension of the intramedullary lesion, as demonstrated by MRI, often does not correlate with the clinical findings during neurologic assessments.13 Numerous studies have shown that SSEPs are useful in assessing cord dysfunction as well as in defining the vertical extension of cord damage in patients with spinal cord lesions.4,14,15 Restuccia et al13 used multisegmental SSEPs in patients with focal lesions of the lumbosacral region to demonstrate that spinal cord dysfunction involves more segmental levels of the spinal cord than those directly demonstrated by MRI. Although abnormal SSEPs findings are not specific for a spinal cord tumor, preoperative electrophysiologic evaluation could be important in these cases for several reasons.14 First, it can evaluate the integrity of the different levels of the spinal cord to define the extension of the lesion. This could provide the surgeon with information on the extent of the lesion and facilitate complete removal. Second, because neurosurgical treatment of these patients is associated with a risk of causing new postoperative neurologic deficits, an objective assessment of spinal cord dysfunction should be available for the purposes of future monitoring. The literature suggests that SSEPs are a sensitive measure of spinal cord dysfunction caused by cervical or lumbar cord tumors.14,15 Although MRI is the procedure of choice for diagnosis and management of spinal neoplasms, MRI is seldom indicated in patients with vague complaints or nonlocalizing signs. This may be particularly relevant in children with intraspinal tumors, in whom the symptoms are often vague. Jabbari and coauthors14 found that 44% of patients with an intraspinal neoplasm who presented only vague signs or had a normal neurologic examination demonstrated abnormal SSEPs. This could result in a delay in definitive radiologic investigation. The method used to test the integrity of the long tracts of the spinal cord, using direct stimulation and recording from the spinal cord, was first described by Japanese investigators.16 A recording electrode can be introduced into the subarachnoid space from the surgical field, or epidural recordings can be performed by percutaneous introduction of the electrode into the epidural space.17 Electrical stimulation to the spinal cord is delivered through a bipolar tube-type electrode placed in the epidural space. Electrical stimuli of 0.2 to 0.3 msec in duration, 3 to 13 mA in strength, and 20 to 50 Hz in frequency are used to elicit SCEPs. The advantages of SCEP recordings are that these responses are largely unaffected by anesthesia and have a larger amplitude when compared with SSEPs recorded from the scalp. Although variations occur from patient to patient, the SCEPs in general consist of a permanent initial spike wave followed by polysynaptic waves. Tamaki et al16 particularly emphasizes the importance of changes in the configuration of the polyphasic wave portion of SCEPs during intramedullary spinal cord surgery, which places the dorsal columns at risk. In contrast to cortically recorded SSEPs, SCEP that are recorded directly from the spinal cord are much less sensitive to the effects of anesthesia; however, there are a few potential disadvantages of recording from the spinal cord. First, the possibility exists of complications related to placing electrodes near the spinal cord.18 Second, there are difficulties in establishing the exact position of the electrodes and in maintaining their position during surgery.5 The SCEPs evoked via spinal cord stimulation have been utilized during the intraoperative monitoring of spinal cord tumors by Koyanagi and colleagues.5 Despite reproducible recordings, this evoked potential technique was not sensitive to mild degrees of neurologic dysfunction, as determined by postoperative clinical evaluation. These authors conclude that SCEP monitoring can be used as an alternative method or in combination with other types of evoked potentials in patients with severe spinal cord lesions who show abnormal SSEPs preoperatively. The authors also propose that in cases where the spinal cord is severely damaged by structural pathology, more than one modality of intraoperative monitoring of the remaining axonal integrity may be required. Historically, recording SSEPs after stimulation of peripheral nerves was the first method introduced for intraoperative monitoring of spinal cord function during operations for scoliosis.19 Based on a review of the literature over the past 5 years, it is clear that this technique is the predominant modality used for intraoperative spinal cord monitoring.6,20–22 The recordings of SSEPs in patients with spinal and spinal cord lesions provide challenges for the technologist and surgeon in terms of interpreting the recorded parameters. This method has undergone several modifications and additions. Classically, SSEPs are usually recorded with surface or needle electrodes from the skin or scalp of the patient; however, when epidural recordings can be made, some authors believe that they can provide better monitoring than surface recordings.23 Deletis and colleagues24 also introduced a modified 8-channel recording electrode placed on the surface of the spinal cord to map dorsal columns. This method is particularly of interest during surgery for intramedullary tumors. Often the anatomy of the dorsal columns is distorted by the tumor; therefore, dorsal column mapping becomes extremely important to identify the midline of the spinal cord and to preserve the fasciculus gracilis and fasciculus cuneatus during the performance of midline myelotomy. Most commonly, electrical stimuli of 0.2 msec in duration, 2 to 3 Hz in frequency, with a strength of three to five times the motor threshold are applied to elicit SSEPs. The electrical stimulation is usually delivered to a peripheral nerve in the upper (median or ulnar nerves) and lower (posterior tibial or common peroneal nerves) extremities. In our center we routinely utilize the following standard setting for the recording of the upper limb SSEP: extracranial electrodes are placed at the Erb’s points (bilaterally, midclavicle area) and C2 vertebra (a single, midline electrode). Cranial recording electrodes are usually positioned at the following sites: scalp electrodes CPz, FPz, CP3, and CP4, left and right mastoid electrodes (using the 10–20 electrode system,25 Fig. 15-2). The lower limb SSEP is usually recorded using the same electrode setting, with the exception of Erb’s points. The SSEPs are recorded repeatedly during surgery using continuous averaging from 200 to 1000 averages. The amplitude and the latency of the most prominent waves are compared with the baseline values during the different stages of surgery. We routinely use transcutaneous needle electrodes that are placed on patients after the induction of anesthesia. In addition to conventional scalp recordings of SSEPs, direct spinal cord recordings above and below the level of lesion could be useful during the surgery for spinal cord lesions. This is particularly useful in cases where spinal cord lesions significantly disrupt the spinal cord pathways involved in the propagation of the SSEPs. For example, during the surgery for a cervicomedullary junction lesion (Fig. 15-3A), intraoperative upper extremity SSEPs were unobtainable from the right side, with a well-defined subcortical N13 component on the left (Fig. 15-3B, Cv2-Fpz recordings). The localization of the lesion (the lesion occupied most of the right nucleus cuneatus) and the clinical neurologic presentation in this patient correlated well with the absence of the N13 component on the right side; however, during the course of the surgery, using subarachnoid electrodes placed after exposure of the spinal cord and caudal brainstem, we were able to obtain evoked potentials confirming the functional integrity of the ascending spinal pathways caudal to the lesion on the right side (Fig. 15-3B, below the lesion recordings). Using subarachnoid recordings from the midline of the spinal cord and the caudal portion of the brainstem (electrodes were positioned below and above the level of the lesion), we were able to obtain well-defined evoked potentials following stimulation of the left median nerve; however, evoked potentials were above the lesion following stimulation of the right median nerve (Fig. 15-3B, above the lesion recordings). It is thus crucial to appreciate the necessity of variations in SSEPs recording and stimulation techniques during the planning of the approach to intraoperative monitoring for spinal/spinal cord neoplasm cases. FIGURE 15-2 The 10–20 system for placement of EEG electrodes: lateral (A) and top (B) views of the patient’s head. Fp, frontal pole position; F, frontal line of electrodes; C, central line of electrodes; P, parietal line of electrodes; O, occipital line. The Cz point is positioned at half the distance between the nasion and the inion (indicated in the diagram). The cortical SSEPs, elicited by median nerve stimulation, can be recorded 7 cm from midline contralaterally and 3 to 4 cm behind the central plane (C4 and C3). For the tibial nerve SSEPs the recording electrodes are usually placed on the midline at 3 cm behind the central plane (Pz). (From Jasper,25 with permission of the International Federation of Clinical Neurophysiology.) FIGURE 15-3 Magnetic resonance imaging (MRI) (A) and intraoperative recordings (B) in a 65-year-old woman with a cervicomedullary junction granuloma. Two consecutive recordings are presented for each channel. A subcortical N13 component of upper extremity SSEPs was unobtainable during the stimulation of the right median nerve with well-defined N13 component obtained with the left median nerve stimulation (Cv2-Fpz recordings). After the exposure of the spinal cord and caudal brainstem, subdural electrodes were placed, which enabled recordings of bilateral evoked potentials from the surface of the spinal cord below the lesion (below lesion recordings); however, only evoked potentials to the stimulation of the left median nerve were present during recording from the surface of the caudal portion of the brainstem (above lesion recordings). Calibration: 5 msec and 1 μV. The use of SSEPs in the intraoperative monitoring of cases with spinal or spinal cord tumors has some limitations. First, baseline SSEPs recordings in some cases cannot be obtained or are of poor quality as a result of severe myelopathy or direct involvement of the dorsal columns.4,14,15,26,27 Jabbari and coauthors14 reported abnormal preoperative SSEPs in up to 76% of patients with intraspinal neoplasms. Second, SSEPs could be lost after the initial dorsal myelotomy, leaving the surgeon without any means of monitoring the functional integrity of the spinal cord if other complementary methods were not used in conjunction with SSEPs. Finally, the most common criticism deals with the delay in processing and averaging SSEPs (3 to 5 minutes), which may result in a potentially preventable neurologic injury being missed. The issue of asymmetrical or distorted baseline SSEPs is illustrated by the case depicted in Fig. 15-4. In this case SSEPs were obtained during surgical resection of a hemangioblastoma within the cervicomedullary junction (Fig. 15-4A,B). Intraoperatively, the patient showed significant asymmetry of the short latency component N13, elicited by stimulation of the right median nerve (Fig. 15-4C,D). There was also a slight increase in latency and a decrease in amplitude of the N20 component evoked by right median nerve stimulation (Fig. 15-4D). At the same time, the amplitude of the Erb’s point potentials was similar on both sides. Awareness by the neurosurgeon and technologist of abnormal SSEPs in these cases is required to interpret these findings during surgery. Prior to the introduction of motor evoked potentials, we were limited in our ability to monitor the integrity of important descending motor tracts. Furthermore, MEPs monitor the integrity of the lateral corticospinal tracts but do not assess ventral spinal pathways. This led to a search for other ways to monitor the integrity of spinal cord structures during neurosurgery. Our center first described the use of sensory-evoked potentials recorded from the human occiput for intraoperative monitoring of the spinal cord.9 Our group showed in a series of experimental animal studies and then in clinical studies that evoked potentials can be reliably recorded from the occiput and that these potentials showed characteristics independent of classical SSEPs.28,29 Moreover, we concluded that this technique allowed monitoring of the physiologic integrity of the ventral tracts of the spinal cord in humans. In our study, conventional SSEPs and cerebellar-evoked potentials (CEPs) were elicited by percutaneous needle stimulation of the right or left posterior tibial nerve in 25 patients.9 Five of these subjects received surgery for intramedullary or extradural tumors of the spinal cord. The CEPs were recorded from the base of the occiput with needle electrodes positioned along the superior nuchal line 2 to 3 cm lateral to the midline (Occ), from intradural needle electrodes, or from the surface electrodes placed directly on the cerebellar hemispheres. In 14 patients we simultaneously recorded SSEPs, bilateral Occ-Fpz responses, and cervical spine (C2-Fpz) to compare occipital and spinal potentials. In one case of a 29-year-old man with an intramedullary ependymoma extending from C3 to T4, we were able to observe independent amplitude and latency shifts while recording SSEPs and CEPs simultaneously (Fig. 15-5A). Baseline recordings of SSEPs and CEPs showed well-defined responses; however, during tumor removal through a dorsal midline myelotomy, the amplitude of the P37-N45 SSEP decreased by 40%, whereas the amplitude of the N33-P40 Occ-Fpz decreased by only 10 to 17%. Moreover, shifts in the latency of the SSEPs were more pronounced in comparison with the latency of the CEPs (Fig. 15-5B). Postoperatively, the patient presented with dorsal column deficits, but with normal power in the lower limbs. A pioneering paper by Merton and Morton30 in 1980 described the first observation and practical aspects of the electrical stimulation of cerebral cortex in intact human subjects. Later MEPs elicited by transcranial electrical or magnetic stimulation have been utilized for intraoperative monitoring in neurosurgery.31–35 Magnetic stimulation of the motor cortex for intraoperative monitoring did not prove practical. First, it is difficult to maintain the position of the coil throughout the surgical procedure. Second, the MEPs evoked by magnetic stimulation are more sensitive and vulnerable to anesthesia.36,37 Presently, three different methods of recording MEPs have found applications for intraoperative monitoring. MEPs could be recorded from the epidural (or subdural) space of the spinal cord, from peripheral nerves (compound nerve action potentials, CNAPs), and finally from muscles (compound muscle action potentials, CMAPs).7,17,38 One of the major advantages of using MEPs during spine or spinal cord surgery is that this technique allows for the direct monitoring of motor pathways that may be impaired independently of the dorsal column tracts that are monitored by SSEPs. In numerous studies, patients have been reported with postoperative motor deficits even when SSEPs were stable during surgery.21,39,40 MEPs have been successfully used during intramedullary spinal cord tumor surgery in adults and children; however, Morota et al8 acknowledged that MEPs are more difficult to monitor in children. The authors suggested that incomplete myelination of the corticospinal pathways and the increased vulnerability of immature neuronal structures to compression could be contributing factors in the difficulty in monitoring of MEPs in children.
Electrophysiologic Testing for Diagnosis and Surgical Monitoring During Resection of Tumors of the Spine, Spinal Cord, and Peripheral Nerves
 Functional Anatomy of the Spinal Cord and Intraoperative Monitoring
Functional Anatomy of the Spinal Cord and Intraoperative Monitoring
 Preoperative Electrophysiologic Diagnosis of Tumors of the Spine Spinal Cord, and Peripheral Nerves
Preoperative Electrophysiologic Diagnosis of Tumors of the Spine Spinal Cord, and Peripheral Nerves
 Intraoperative Monitoring Techniques During Spinal/Spinal Cord Surgery
Intraoperative Monitoring Techniques During Spinal/Spinal Cord Surgery
Spinal Cord-Evoked Potentials (SCEPs)
Somatosensory-Evoked Potentials (SSEPs)
Cerebellar Evoked Potentials
Motor Evoked Potentials (MEPs)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree