Chapter 22 Endoscopic Endonasal Pituitary and Skull Base Surgery
Neuroendoscopy was first implemented almost a century ago for choroid plexus surgery in a patient with hydrocephalus. General enthusiasm for ventricular endoscopy experienced an initial decline with the advent of ventricular shunt systems but was later revived for third ventriculostomies in selected patients. The first reported use of the endoscope specifically for trans-sphenoidal surgery was in a sublabial approach by Guiot and colleagues in 1963.1 However, the general advancement of intracranial and spinal neuroendoscopy continued to be limited, in part trumped by historical developments in neuroimaging and microneurosurgery. Yet in the past three decades, a few pioneering endoscopic neurosurgeons continued to expand and refine the use of neuroendoscopy in endoscope-assisted microsurgery, endonasal trans-sphenoidal surgery, ventricular tumor surgery, extra-axial intracranial surgery, intra-axial brain surgery with stereotactic guidance, and spinal surgery. These advances were accompanied by concurrent technological developments in endoscopic optics, video-imaging systems, endoscopic accessory attachments for neurosurgical applications, specialized neuroendoscopic surgical instruments, radiologic imaging, and compatible frameless stereotactic image-guided systems. As neuroendoscopic surgical techniques and equipment have co-evolved, the addition of neuroendoscopy to the repertoire of the modern neurosurgeon has become increasingly practical. Of note, general interest has noticeably grown for the use of neuroendoscopy in endonasal trans-sphenoidal pituitary surgery, and the common use of neuroendoscopy in pituitary surgery became truly practical in recent years with the development of commercially-available neuroendoscopic equipment.
Although the removal of pituitary tumors completely through endoscopic visualization via an endonasal route has been a relatively recent development, the use of the endonasal pathway itself was initially reported in 1909 by Hirsch who performed his first pituitary surgery in Vienna by approaching the sella through an endonasal route using multiple-staged sinonasal operations with naked-eye visualization. Despite his first endonasal trans-sphenoidal surgery having reported success, Hirsch subsequently converted to a trans-septal submucosal approach, possibly due to fear of surgical infection through such a wide communication made between the nasal and the cranial cavity. Griffith and Veerapen revisited the endonasal approach in 1987, with insertion of a trans-sphenoidal retractor through the natural nasal airway to the sphenoid rostrum for microscopic pituitary surgery.2 In 1994, Cooke and Jones reported the lack of sinonasal and dental complications when an endonasal route was adopted for microscopic pituitary surgery.3 But the most significant progression of the traditional sublabial and transfixional-trans-septal approaches to the direct endonasal route was highly facilitated by the neuroendoscope, with the initial use of sinonasal endoscopy in Europe four decades ago. In the field of Otolaryngology, the introduction of endoscopic sinus surgery to the United States kindled an evolution in surgical techniques such that endoscopic sinus surgery rapidly replaced many forms of conventional sinus surgery, with radical changes in concepts of sinonasal pathophysiology and associated treatments aided by endoscopic exploration. Rather than stripping the infected sinus mucosa as was done in conventional sinus surgery, endoscopic sinus surgery aimed to restore physiologic mucous drainage merely by eliminating obstructive pathoanatomy at sinus ostia via the endonasal route and became popularized as functional endoscopic sinus surgery (FESS). Successful advances in sinonasal endoscopy then enhanced interest in the use of endoscopy for trans-sphenoidal surgery. Endoscopic trans-sphenoidal surgery started with guidance during simple biopsy of a sellar lesion, and then evolved to assist visualization during insertion of trans-sphenoidal retractors or during microscopic removal of pituitary adenomas, and eventually the pure form of endoscopic endonasal pituitary tumor surgery emerged.4–12
This chapter describes endoscopic endonasal trans-sphenoidal surgery (EE-TS) along with related endoscopic endonasal approaches to the midline skull base such as the anterior cranial fossa (EE-ACF), optic nerve or cavernous sinus (EE-CS), pterygoid fossa or petrous apex (EE-Pterygoid or EE-Petrous), clivus or posterior fossa (EE-PFossa), and craniocervical junction (EE-CC junction). EE-TS is not merely endoscope-assisted microscopic surgery but is rather an operation done completely with an endoscope without any trans-sphenoidal retractor or nasal speculum, eliminating the need for postoperative nasal packing or other adjuncts. The physical nature of an endoscope with its optics at the tip and slender shaft allows simple access to the sella through the natural nasal air pathway via a nostril. EE-TS uses an endonasal route to the rostrum of the sphenoid sinus with an anterior sphenoidotomy about 1 to 1.5 cm in diameter. The wide-angled panoramic view, angled-lens views, and a close-up zoom-in view provide optical advantages with distinct visualization at the surgical target site. The application of principles and anatomy in EE-TS was extended to the surgical treatment of midline skull base pathologies (from the anterior cranial fossa to the clivus and posterior fossa along with the craniocervical junction) and paramedian skull base pathologies (from the optic nerve and cavernous sinus regions to the pterygoid fossa and petrous tip). Endoscopic endonasal techniques can potentially be applied to nearly any lesion within approximately 2-cm width of the midline skull base from the crista galli at the anterior-superior skull base to the foramen magnum and atlantoaxial region at the posterior-inferior skull base.13–23
Pertinent Sinonasal Anatomy
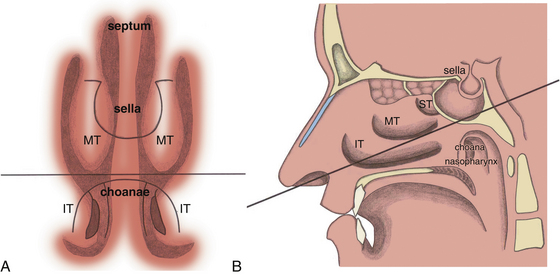
The nasal cavity itself is bordered medially by the nasal septum (comprised of the septal cartilage, perpendicular plate of the ethmoid bone, and the vomer); superiorly by the cribriform plate of the ethmoid bone and bridge of the nose (consisting of the nasal portion of the frontal bone, nasal bone, and frontal process of the maxilla); inferiorly by the floor of the nasal cavity (involving the palatine process of the maxilla and the horizontal plate of the palatine bone); and conchae or turbinates laterally (inferior, middle, superior, and sometimes supreme turbinates). The superior and middle conchae (along with the occasional supreme concha) are components of the ethmoid bone, whereas the inferior concha is a separate bone. The EE-TS procedure traverses the region medial to the middle turbinate, between the middle turbinate and the nasal septum, on the way to the sphenoid sinus then the pituitary fossa at the sella turcica. Nasal septal deviation is not an uncommon phenomenon, such that often the larger nasal cavity is selected as the route for EE-TS based on preoperative imaging and intraoperative visualization.
As mentioned, there can be significant variability in the structure of the ethmoid sinus, which is sometimes termed the ethmoid labyrinth, with the ethmoid air cells having some surgically pertinent variations for EE-TS. The agger nasi is a mound or prominence on the anterior-lateral aspect of the nasal cavity formed by mucous membrane covering the ethmoidal crest of the maxilla near the anterior aspect of the middle turbinate. The agger nasi cells are the most anterior ethmoidal cells located just anterior and lateral to the nasofrontal recess and can sometimes be involved in frontal sinus outflow obstruction. For EE-TS, the surgeon should be aware that a hyperpneumatized agger nasi cell can occasionally present as a bulge that mimics the anterior view of a turbinate. Haller cells (infraorbital ethmoid air cells or maxilloethmoidal cells) are closely related to the ethmoid infundibulum along the medial roof of the maxillary sinus, inferior-lateral to the ethmoid bulla, and extend into the inferomedial orbital floor at the inferior margin of the lamina papyracea. Since Haller cells are quite lateral, these usually do not present a problem during EE-TS, although their proximity to the ethmoid infundibulum can result in inadvertent orbital entry during FESS if not recognized. Onodi cells (sphenoethmoidal cells) are posterior ethmoidal air cells that can project superiorly into the sphenoid sinus towards the lateral side and can potentially be confused with a septated region of the sphenoid sinus. The optic nerve and/or internal carotid artery can bulge into Onodi cells instead of the sphenoid sinus proper, or occasionally may have either partial or complete bony dehiscence at the sphenoid sinus, presenting risk for injury during surgery. It is also possible to have extensive pneumatization of the anterior clinoid process at the optico-carotid recess (OCR), which can also expose the optic nerve and internal carotid artery to additional risk. It is also possible for patients who have undergone previous trans-sphenoidal surgery to have a mucocele at the region of the sphenoid sinus, which can also potentially lead to intraoperative confusion, although this is usually recognizable on the preoperative MRI with the mucocele usually having a spherical type of shape and bright T2 signal such that it can be anticipated intraoperatively.
When the sphenoid sinus is entered with an endoscope, the complex anatomy is visualized in a panoramic fashion. The clival indentation is seen at the bottom midline, the bony protuberances covering the internal carotid arteries are lateral to the clival indentation, the sella is at the center, the cavernous sinuses are seen lateral to the sella, the tuberculum sella is at the top, and the bony protuberances of the optic nerves are seen laterally. Surgical landmarks for endoscopic endonasal pituitary surgery consist of the choanae and nasopharynx along with the inferior margin of the middle turbinate. The choana at the anterior-superior entry of the nasopharynx is always a useful landmark in order to confirm the middle turbinate. The extended line along the inferior margin of the middle turbinate leads to the region approximately 1 cm inferior to the sellar floor. Although the sphenoid sinus ostium at the sphenoethmoidal recess may also be visible under the endoscope, it may not always be easily identifiable or precisely consistent in its relationship to the sella. Thus, the sphenoid sinus ostium can be regarded as an inconsistent surgical landmark, whereas the choana and inferior margin of the middle turbinate tend to be quite consistent. Anterior sphenoidotomy measuring approximately 1.5 cm in diameter is performed at the rostrum of the sphenoid sinus at a location rostral to an extended line from the inferior margin of the middle turbinate (Fig. 22-1A and B).
The nasal mucosa is predominantly supplied by the sphenopalatine artery, with contributions from the greater palatine artery, branches of the facial artery, and the anterior and posterior ethmoidal arteries. The posterior septal artery arises from the sphenopalatine branch of the internal maxillary artery and passes to the posterior nasal septum at the inferior-medial aspect of the middle turbinate posterior margin. When surgical access is obtained between the middle turbinate and nasal septum for an anterior sphenoidotomy, the posterior septal artery often requires coagulation and division to prevent unwanted intraoperative or postoperative nasal bleeding. Delayed copious nasal bleeding after trans-sphenoidal surgery usually arises from rebleeding of the posterior septal artery.
For endoscopic endonasal approach to the anterior cranial fossa (EE-ACF), the region of the ethmoid roof with the cribriform plate and the fovea ethmoidalis should be studied on preoperative imaging. Keros categorized three types of skull base conformations along a spectrum depending on the depth of the olfactory sulcus and corresponding height of the lateral lamella, with Type 1 having 1-3 mm depth, Type 2 having 3-7 mm depth, and Type 3 having 7-16 mm depth such that the ethmoid roof is significantly higher than the cribriform plate.24 The Keros classification is more pertinent for ethmoid sinus surgery to avoid inadvertent entry through the thin lateral lamella of the cribriform plate, especially for a deep olfactory sulcus. However, for EE-ACF or EE-CS (via the middle meatal approach with posterior ethmoidectomy), one should be aware of the potential individual variations in depth of the olfactory sulcus and the specific anatomic configuration should be noted preoperatively to assist with optimal intraoperative maneuvering. In the same region, the ethmoidal sulcus is the groove in the lateral lamella for the anterior ethmoidal artery, which branches from the ophthalmic artery and enters from the orbit in a bony canal in the ethmoid roof (anterior ethmoidal canal) or just below the level of the ethmoid roof to course anteromedially with the anterior ethmoidal nerve and to penetrate the lateral lamellae to supply the dura in the region of the olfactory sulcus. The posterior ethmoidal artery traverses the posterior ethmoidal canal within a 3 mm planar region above the cribriform plate. The anterior and posterior ethmoidal arteries usually provide the major blood supply for olfactory groove or planum sphenoidale meningiomas at the skull base.
Optical Advantages of an Endoscope
Wide-Angled Panoramic View
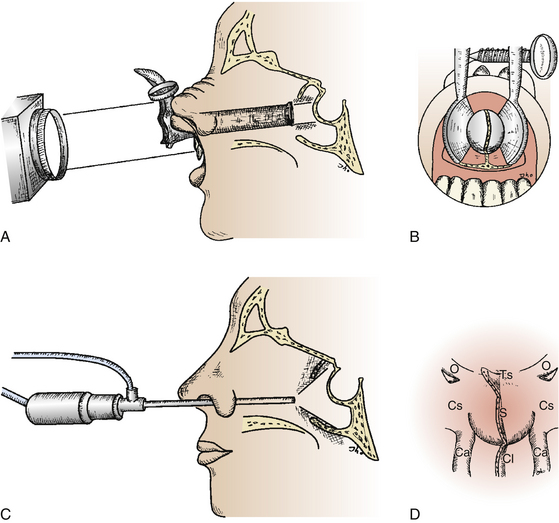
As trans-sphenoidal pituitary surgery began its evolvement in the 19th century, one major advance was the adoption of the operating microscope in the 1960’s. The use of the endoscope for pituitary tumor resection represents another significant advancement. Whereas the operating microscope provides a magnified view of a limited portion of the sella through a narrow corridor revealed by the trans-sphenoidal retractor, an endoscope can physically enter into the sphenoid sinus and provide a wide-angled panoramic view with zooming capability. An operating microscope renders a tubular parallel beam view, but an endoscope shows a diverging flask-shaped wide-angled view (Fig. 22-2A to D). This wide-angled panoramic view is particularly useful for pituitary tumor surgery because it allows excellent anatomical visualization at the posterior wall of the sphenoid sinus. However, it must be recognized that the endoscopic view renders a fish-eye effect with maximum magnification at the center and relative contraction at the periphery with visualization of a wide anatomical area. In the well-pneumatized sphenoid sinus, the sella is readily recognizable at the center of the surgical view, and a panoramic image of the surrounding anatomy at the posterior wall of the sphenoid sinus is revealed under direct endoscopic view. Unless the region of the sella is not pneumatized or the patient is a complicated reoperation case, the use of fluoroscopic roentgenogram is not necessary since endoscopic visualization can adequately reveal the distinct surgical anatomy.
Angled-Lens View
The angled-lens endoscopic view provides direct visualization of the anatomical corners such as the suprasellar area or towards the cavernous sinus. These views can be of great assistance even if an endoscope is only used as an adjunctive tool during conventional microscopic surgery. Operating under an angled-lens endoscopic view requires specially designed surgical tools and advanced endoscopic surgical skills, particularly for the 70-degree-lens endoscope. As an angled-lens endoscope is rotated towards the surgical target, various anatomical corners can be visualized from the floor of the sella to the medial wall of the cavernous sinus and towards the suprasellar region. A fiberoptic endoscope can sometimes be used to inspect anatomical corners involving curved routes. This angled view is advantageous when large suprasellar macroadenomas are to be removed. It also allows clear visualization at the medial wall of the cavernous sinus when the lateral margin of a sellar tumor abutting the cavernous sinus is dissected away or when tumor tissue invading the cavernous sinus is to be removed under direct visualization. Although pituitary adenomas with significant invasion of the cavernous sinus were once generally regarded as inoperable, EE-TS allows safe access to the cavernous sinus for tumor removal. Angled views allow for direct surgical access to the pterygoid fossa, anterior cranial fossa, clivus, and posterior cranial fossa in addition to the cavernous sinus (Fig. 22-3A and B).
Surgical Procedure
Surgical Instruments
Appropriate surgical equipment is necessary to perform optimal endoscopic pituitary surgery. Attempting an endoscopic operation of this nature with a borrowed otolaryngologic endoscope can potentially result in frustration. Endoscopic surgical techniques are quite different from those of microscopic surgery. Being well-trained in microscopic surgery does not preclude the need for practice in endoscopy. The required surgical instruments are endoscopes with 0-, 30-, and 70-degree lenses (Fig. 22-4A) and their appendages, including a video-imaging system and light source connections, an endoscope lens-cleansing device, a rigid endoscope holder, and various other surgical instruments specifically designed for endoscopic pituitary surgery. The length of an endoscope must be 18 cm or longer. When an 18 cm-long endoscope was used for removal of a posterior fossa tumor through an endonasal transclival approach, it proved to be marginally short and restricted the surgeon’s operating space between the endoscopic appendages and the patient’s face. Fluoroscopic guidance, which was used in earlier patients, is no longer used in routine pituitary surgery. It is rarely but occasionally used for anterior or posterior cranial fossa surgery, and for pituitary tumor patients in which complexity of the sinonasal anatomy is anticipated.
An endoscopic lens-cleansing device is required to cleanse the lens so that the surgeon can operate without interruption (Fig. 22-4B). The device consists of a disposable irrigation tube, which has a loop of tubing passed through a battery-powered rotary device. The irrigation tube is connected into a warmed saline bag (the temperature prevents lens fogging), which is hung on a pole, and the motor-powered irrigation device is controlled by a foot pedal to flush saline forward. When the foot pedal is released, the motor reverses its rotary direction and draws the saline back for 1 to 2 seconds. The forward flow of irrigation saline cleans the lens, and the reverse flow clears water bubbles at the tip of the endoscope. Although this device is not yet perfect, it helps the surgeon significantly in the task of keeping the endoscope lens clean in order to preserve the optimal technical continuity and flow of the procedure.
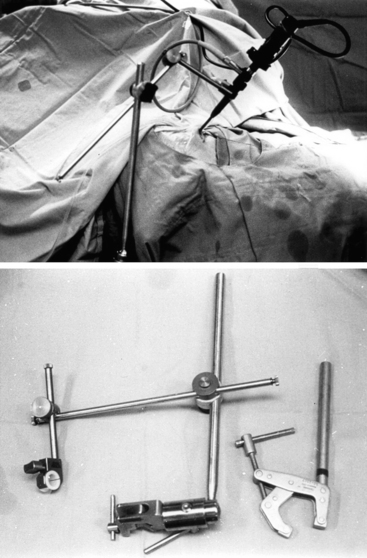
Appropriate endoscope holders help provide stability of the visual image and bimanual instrument use during portions of the case during which this is optimal, such as drilling and the majority of tumor removal (Fig. 22-5). Although there are portions of surgery during which dynamic visualization is important, such as the initial approach to the sphenoid sinus rostrum and final visualization of any tumor remnants at anatomic corners of the sella, endoscope holders provide camera stability akin to a video camera tripod during appropriate segments of surgery and does not require the presence of a trained assistant to constantly drive or hold the endoscope camera. The endoscope holder should also provide rigid fixation of the endoscope while also allowing easy transition between stable fixation and manual dynamic steering. The holding terminal is necessarily compact and slender so as to render adequate operating space around the endoscope shaft for the surgeon to maneuver surgical instruments. We routinely use a customized manual endoscope holder specifically designed for EE-TS, in contrast to a nitrogen-powered holder called the Mitaka Point Setter (Mitaka USA; Park City, Utah) for our endoscopic transcranial approaches, and a nitrogen-powered holder called Unitrac (Aesculap; Tuttlingen, Germany) for our endoscopic spine surgery. Manual holders have multiple joints that are tightened by hand to set the final position, but only a single joint has to be loosened during a case to transition from endoscope fixation to manual driving or back. Manual holders are highly stable without significant constraints in range-of-motion or disadvantage of the settling phenomenon, but the configuration can purposefully be set such that the range-of-motion of the final joint is constrained to maintain the trajectory of the endonasal route for EE-TS and depth adjustments only require the simple untightening of a single joint. Nitrogen-powered holders are more expensive but conveniently provide release and tightening using a single button. The Mitaka Point Setter provides excellent stability and minimal settling phenomenon but is limited by the constrained range-of-motion to cases that require only minor maneuvering of the endoscope holder. The Unitrac is stable and highly maneuverable but displays significant settling phenomenon such that the final endoscope position sinks somewhat with gravity before locking in final position. Other endoscope holders are still in development to attempt to maximize the strengths and minimize the weaknesses of the various endoscope holders.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree