21
Endovascular Treatment of Posterior Circulation Aneurysms
Aneurysms of the posterior circulation account for roughly 20% of all intracranial aneurysms, with the majority occurring at the basilar apex followed by the origin of the posterior inferior cerebellar artery (PICA).1 Less common are aneurysms of the posterior cerebral arteries (PCAs), the anterior inferior cerebellar arteries (AICAs), the superior cerebellar arteries (SCAs), the vertebrobasilar junction, and the basilar artery (BA) trunk. The deep location of these aneurysms and intimate relationships to critical neurovascular structures explain the relatively high morbidity and mortality related to their surgical treatment.2–4 Over the past 15 years, endovascular techniques have evolved from an unproven surgical alternative to the treatment of choice for posterior circulation aneurysms at many centers. As we shall discuss in this chapter, endovascular treatment of aneurysms is no longer limited to simple “coiling” of an aneurysm. New techniques have expanded the role of endovascular treatment for intracranial aneurysms and have established endovascular therapy as the first-line treatment for many posterior circulation lesions. That this bias is not simply unique to our institution but rather a disseminated concept is evident by the recent International Subarachnoid Aneurysm Trial (ISAT), in which only 2.7% of the enrolled cases involved aneurysms of the posterior circulation.5,6
♦ Selection of Patients
As mentioned above, the location of an aneurysm in the posterior fossa by itself presents favorably for endovascular therapy over surgery. At our institution, we consider coil embolization as first-line therapy for most posterior circulation aneurysms that warrant treatment. Surgery is considered only if all current endovascular techniques are exhausted and unsuccessful. This approach is much different than our approach to anterior circulation aneurysms, where we consider initially both treatment options. Clinical as well as anatomic factors must be considered in the selection of posterior circulation aneurysms for endovascular therapy. Advanced patient age, significant medical comorbidities, poor clinical grade of the ruptured lesions, and associated vasospasm also weigh favorably toward endovascular therapy.
♦ Fundamentals of Endovascular Technique
A complete review of endovascular technique is beyond the scope of this chapter. This section outlines the major steps of coil embolization of aneurysms as performed at our institution. Direct coil embolization without the use of any supporting devices such as balloons or stents can be accomplished in the vast majority of intracranial aneurysms. The ideal aneurysm morphology for coiling is a dome-to-neck ratio of >2 and a neck diameter of less than 5 mm.7 Endovascular treatment of aneurysms ideally should be quick and straightforward to minimize complications. In contrast to open surgery, the endovascular approach to most intracranial aneurysms is generally the same, regardless of anatomic location. Of course, there are some institutional variations in technique, but the general principles of endovascular therapy are uniform. Endovascular procedures are routinely performed in our neuroangiography suite, with the patient under light intravenous sedation and analgesia. In this fashion, we can assess the patient’s neurologic status periodically. We reserve general anesthesia for patients with altered mental status or for those in whom light sedation is insufficient to reduce anxiety or movement. We are also more likely to use general anesthesia in patients with subarachnoid hemorrhage (SAH) because of their headache or altered mental status. Owing to the risk of intraprocedural aneurysm rupture, we do not hesitate to revert to general anesthesia for patients in whom conscious sedation is insufficient. All endovascular procedures are performed under continuous hemodynamic monitoring.
Ventriculostomy is used commonly to treat acute hydrocephalus in patients with aneurysmal SAH. The routine use of anticoagulants (see below) and antiplatelet agents during and after endovascular therapy may increase the hemorrhagic complications of such bedside procedures. Therefore, our threshold to perform a ventriculostomy is lower in patients who undergo coiling. If ventriculostomy is indicated, it is best done before the endovascular procedure rather than after it.
To reduce thromboembolic complications during aneurysm coiling, systemic anticoagulation is induced with intravenous heparin dosed according to body weight. An activated coagulation time of 250 to 300 seconds is achieved before we advance the microcatheters. For coiling of ruptured aneurysms, heparinization is usually delayed until the first coil has been deployed and the aneurysm dome is secured. In some cases where microcatheterization is anticipated to be difficult and prolonged, the patient initially receives a half dose of heparin, with the other half given immediately after placement of the first coil.
Arterial access is usually obtained through the common femoral artery where a sheath is introduced with a modified Seldinger technique. A guiding catheter is used to navigate the aorta and engage the extracranial vertebral arteries (VAs). Occasionally, these arteries may be difficult to access via the aortic arch because of tortuosity of the subclavian arteries, diffuse atherosclerotic disease, or oblique origins from the subclavian arteries. In these cases, we do not hesitate to use a radial or brachial artery approach. Digital cerebral angiography is then performed, and projections are found that optimally delineate the aneurysm neck with the relation to the parent blood vessel and neighboring branches. In recent years, rotational three-dimensional views have been useful in finding the optimum projection.
Using road-map guidance, the cerebral vessels are navigated with a microcatheter over a microwire, and the aneurysm is catheterized either directly with the microcatheter or over the microwire. In general, the vertebrobasilar system is less tortuous than the anterior circulation, and endovascular navigation is easier. This is particularly true for the BA, thus making microcatheterization of most basilar tip aneurysms relatively straightforward. After verification on two projections of the location of the microcatheter, platinum microcoils of different conformation, shapes, sizes, and stiffness are selected. The selected coils are delivered through the microcatheter, and the coils are detached when the position is evaluated to be appropriate and stable. If a chosen coil does not conform to the aneurysm shape or herniates into the parent vessel after several attempts, the coil is removed and a different one is chosen.
Before detachment, attention is focused to confirm the absence of parent vessel occlusion, thrombus, stenosis, or contrast extravasation. The endpoint of the procedure is dense packing of the aneurysm until additional coils cannot be placed. After microcatheter removal, final views should confirm the absence of aneurysm filling and any vascular complication related to the therapy (e.g., parent vessel stenosis, distal embolization). Final cervical angiographic runs should also be acquired to search for any iatrogenic dissection occurring during the guide-catheter positioning.
♦ Expanded Techniques
The vast majority of embolized aneurysms can be coiled primarily as described above. However, certain large and broad-necked lesions cannot be treated with coiling alone. The unfavorable morphology of these aneurysms reduces the possibility of dense coil packing. The use of three-dimensional coils, balloons, and stents has expanded the endovascular treatment of large and complex aneurysms.
Three-dimensional coils were introduced in 1999. These soft coils have a three-dimensional memory so that they bend randomly during their deployment to conform to the shape of the aneurysm.8,9 This design allows these coils to bridge the neck of the aneurysm and provide a scaffold within which additional coils can be packed. As a result, the coil packing density can be increased in wide-necked aneurysms without prolapse of the coils into the parent vessel. After placement of several three-dimensional coils, the remaining open spaces of the aneurysm can be filled with two-dimensional coils. Complete aneurysm occlusion can be achieved in more than two thirds of aneurysms with wide necks, provided the dome-to-neck ratio is greater than 1.5.9 For aneurysms with very wide necks or dome-to-neck ratios less than 1.5, balloon or stent assistance may be necessary.
Balloon-assisted coiling involves intermittent inflation of a balloon positioned across the wide neck of an aneurysm.10 Because the inflated balloon is occlusive of the parent vessel, it is deflated after coil detachment. The purpose of the inflated balloon is to prevent herniation of the coil as it is deposited into the aneurysm (Fig. 21.1). It must be emphasized that balloon assistance may not affect packing densities and may not improve aneurysm recurrence rates. The complications of balloon-assisted coiling range were initially thought to be few, but complication rates as high as 14% have been described recently.11 Despite the increased complication rates with balloon as well as stent assistance, these adjunctive techniques still compare favorably to surgical results for posterior fossa aneurysms. Some of the increased complication rate is inherent to the complexity of the class of aneurysms that these adjuncts are designed to treat. The increased risks of using these adjunctive techniques must be weighed against the risk of treating these aneurysms surgically.
The concept of stent-assisted coiling is relatively straightforward and elegant. The stent is deployed across the wide neck of the aneurysm; microcatheterization of the aneurysm itself is then accomplished through the stent mesh, which prevents coil herniation into the parent vessel. Initially, balloon-mounted coronary stents were the only ones available for intracranial use. However, these stents can be difficult to navigate in the tortuous intracranial vessels. Furthermore, their stiffness and pressure-dependent deployment carries substantial risk of injury to the thin-walled cerebral vessels.
Recently, self-expanding stents have been introduced for intracranial use. The Neuroform (Boston Scientific, Fremont, CA), a nickel-titanium alloy stent with a low profile and high porosity, is currently the only stent approved in the United States for intracranial aneurysm treatment. Although this stent is more navigable than the balloon-mounted stents, extremely tortuous arteries still present a formidable challenge for delivery. Stents have advantages over balloon assistance in that they offer a permanent scaffold to prevent coil herniation. The struts of the Neuroform stent do not appear to occlude adjacent branch arteries due to the open cell design of the stent.12
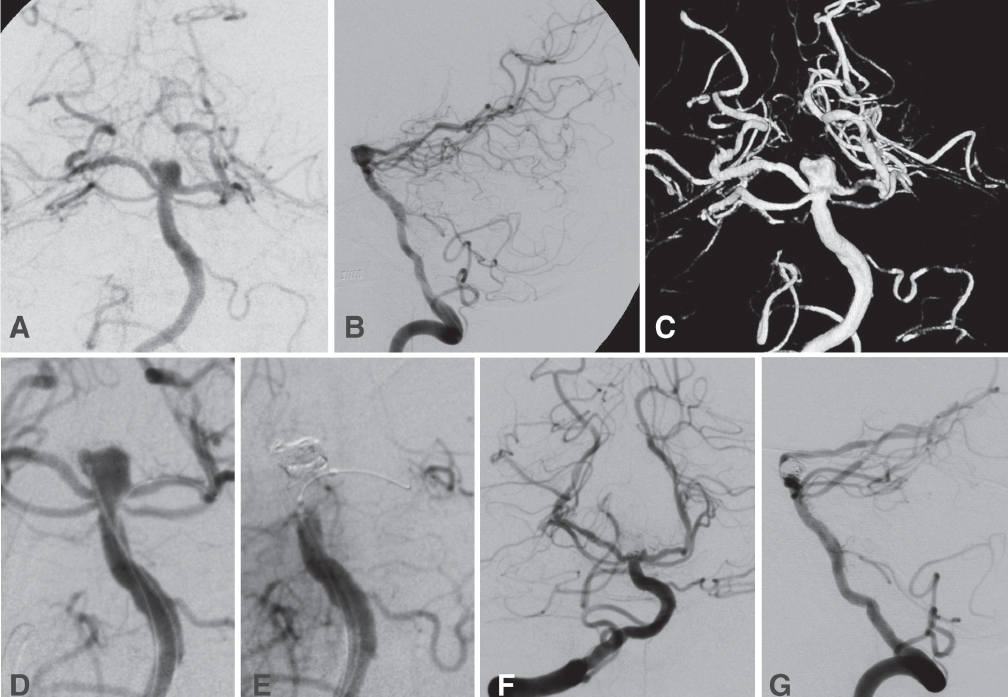
Fig. 21.1 This 60-year-old man presented in a comatose condition after a subarachnoid hemorrhage (SAH). His clinical status improved to Hunt-Hess grade IV after ventriculostomy. Angiography (A, anteroposterior [AP] projection; B, lateral; and C, three-dimensional reconstruction), revealed a broad-necked basilar tip aneurysm incorporating the left posterior cerebral artery (PCA) origin in the neck. Stent-assisted coiling was not done because of the acute rupture. Instead, we proceeded with balloon-assisted coiling of the aneurysm. (D) The AP view shows positioning of the microcatheter in the aneurysm. The deflated balloon is positioned in the distal basilar artery (BA) and proximal left PCA. (E) Intermittent inflation of the balloon confirms occlusion of the distal BA as the coils are deposited in the aneurysm. Final views (F, AP; and G, lateral) show preservation of the left PCA and 95% occlusion of the aneurysm.
Experimentally, stents have been shown to divert blood from the inflow zone of the aneurysm, thus promoting thrombosis within the aneurysm.13 This property can be applied directly to side-wall aneurysms, such as basilar trunk or vertebrobasilar junction aneurysms (Fig. 21.2).14 For such lesions, stenting alone across the aneurysm neck without coiling may be enough to treat the lesion. Despite the problems of balloon-mounted stents, coronary stents have low porosity and have an advantage of impeding aneurysm filling and promoting thrombosis of the lesion. We have also used various combinations of stents within stents to decrease the aneurysm inflow further.
Potential complications of stenting include suboptimal positioning, increased thromboembolism and vessel injury inherent to placing additional devices into the intracranial vasculature, and delayed thrombosis of the stent. Furthermore, the natural history of stented intracranial lesions is not known, and clinical and radiologic surveillance for in-stent stenosis is necessary. We typically maintain patients treated with stents on both aspirin (325 mg daily) and clopidogrel (75 mg daily) for 1 month and then aspirin alone indefinitely thereafter. Overall, the results from stent-assisted coiling are favorable. The rate of technical success associated with stent-assisted coiling has been very high, approaching or greater than 90% in our experience and in published reports.15–17 Complication rates, mostly thromboembolic, have approached 25%, but once again this is partially reflective of the high-risk aneurysms for which this treatment modality has been developed.15 Even with stent-assistance, a recanalization rate of approximately 25% has been observed, but this is comparable to the rate of recanalization observed with direct coiling of small aneurysms.16
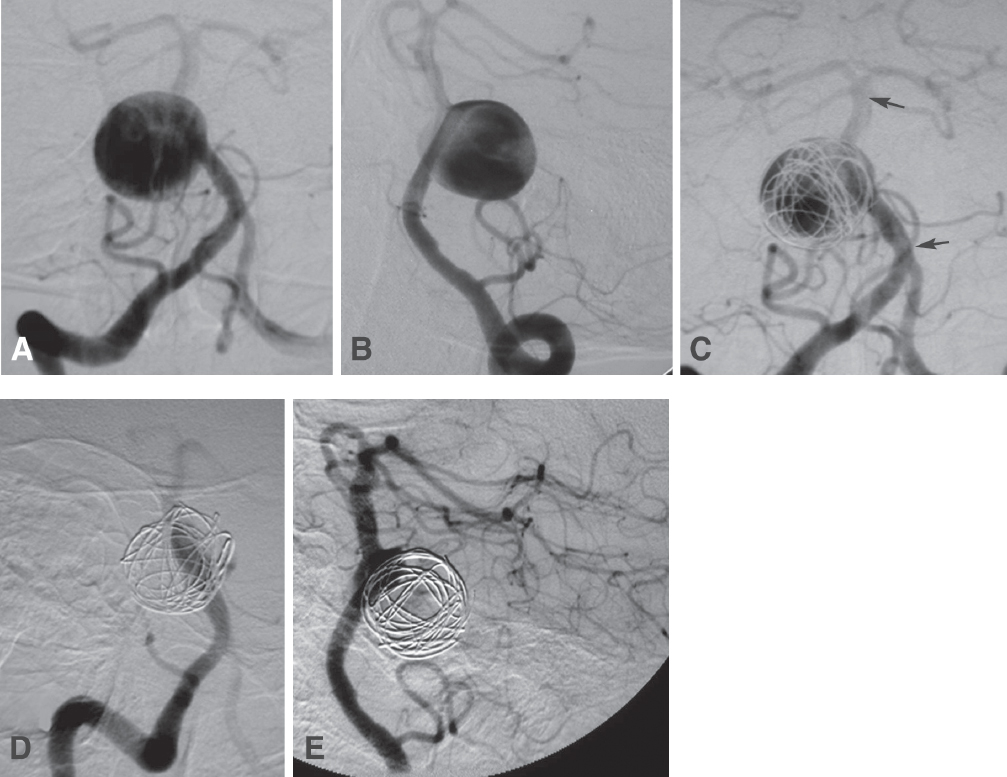
Fig. 21.2 An 82-year-old woman presented with headaches, diplopia from right sixth nerve palsy, and right hemifacial spasm. Views of the right vertebral artery (VA) injection (A, AP; and B, oblique) show a giant aneurysm arising from the basilar trunk. This side-wall aneurysm was treated with placement of two overlapping stents across its neck to reduce flow into the lesion (C, AP view; stents span the segment between the arrows). Two coils were placed in the aneurysm to help induce thrombosis. Views (D, AP; and E, lateral) 1 month later showed that only the aneurysm neck was filling. The patient’s sixth cranial nerve palsy persisted, but her headaches and hemifacial spasm had resolved.
Practical considerations may restrict the role of stenting in the setting of an SAH (Fig. 21.3). The rigorous use of antiplatelet agents required by stenting may impede adjunctive procedures such as ventriculostomy (as described previously), cerebrospinal fluid shunting, tracheostomy, and gastrostomy. These issues are not theoretical because the patients needing these procedures are usually the poor-grade patients selected for endovascular therapy in the first place. One strategy in these patients may be to secure the fundus of the wide-necked aneurysm with direct coil embolization acutely, and then completing the embolization in a delayed fashion with stent assistance. It has been shown that occlusion of the dome and fundus of a ruptured aneurysm may be protective in the short term after an SAH.18 A small neck remnant is usually acceptable in terms of securing the aneurysm. In fact, a small neck remnant after initial coiling may lead to complete aneurysm occlusion due to progressive thrombosis in some cases. Of course, residual aneurysms need to be followed closely, as the risk of delayed rerupture can be as much as 5%.19 Our follow-up strategy for coiled aneurysms is control angiography after 6 months and then at 12 to 18 months if a residual neck is noted. The size of the residual neck determines the timing of the second follow-up angiogram. Aneurysms that remain completely occluded at the first follow-up are usually evaluated thereafter with annual magnetic resonance angiography.
♦ Deconstructive Approach
If the above strategies can be considered reconstructive endovascular techniques, then, for completion, we shall mention deconstructive techniques. This technique involves the endovascular sacrifice of the parent vessel. This strategy is usually reserved for giant or fusiform aneurysms that cannot be treated using conventional endovascular techniques (see below). Occlusion of the VA (or, rarely, the BA) may alter the hemodynamics of the aneurysm and promote aneurysm thrombosis. Endovascular vessel occlusion, originally done with detachable balloons,20,21 is now accomplished usually with detachable coils.
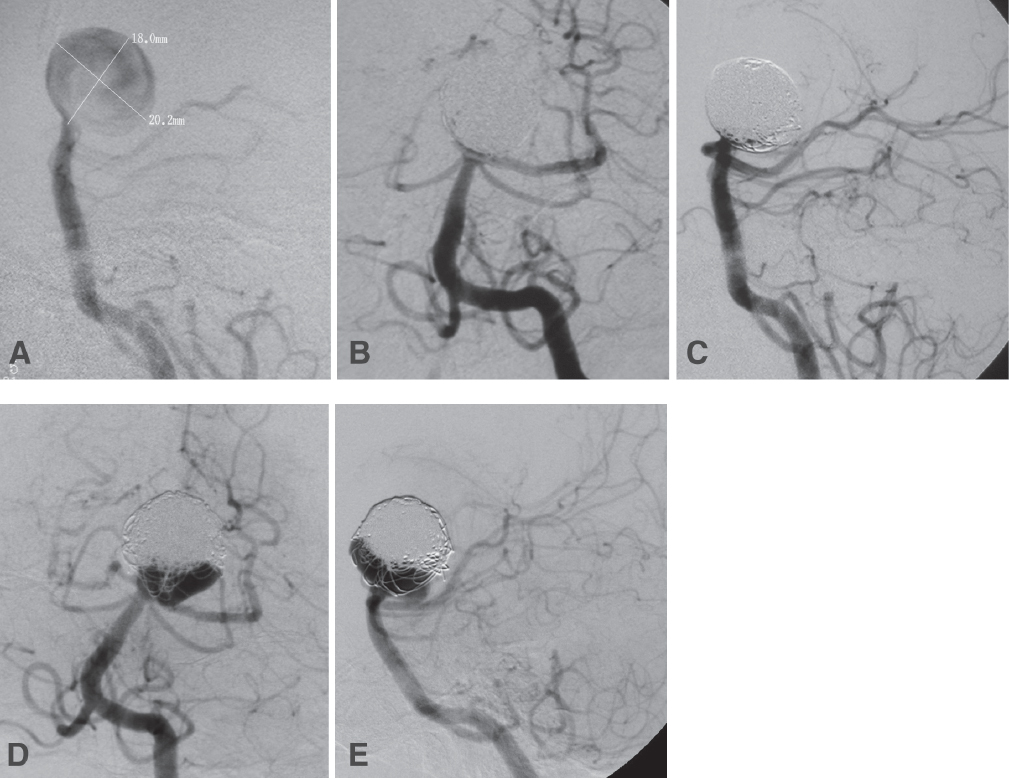
Fig. 21.3 A 69-year-old woman presented with severe headache and lethargy from diffuse intraventricular hemorrhage. (A) Lateral projection of a left VA injection shows a large spherical basilar tip aneurysm. The right PCA filled from the anterior circulation. This aneurysm was treated with direct coiling without stenting because of the acute rupture. Views after coiling (B, AP; and C, lateral) show complete occlusion of the lesion. The patient recovered well and was independent. Angiographic follow-up at 6 months (D, AP view) demonstrated significant recanalization of the aneurysm. The aneurysm was then recoiled electively with stent assistance (E, lateral view). The distal end of the stent was positioned in the left PCA.
Prior to permanent vessel occlusion, balloon test occlusion is usually performed as a diagnostic procedure. An understanding of the collateral supply to the cerebral vascular territory at risk is a prerequisite to vessel sacrifice. A successful balloon test occlusion offers reassurance, but it does not guarantee against stroke after vessel sacrifice. Nonetheless, deconstructive approaches are effective in over 60% of inoperable aneurysms in the posterior circulation.22 Ischemic complications that result from deconstructive approaches are probably due to thromboembolism or failure of collateral circulation. To reduce this risk, endovascular occlusion may be an adjunct to surgical bypass. Despite parent vessel occlusion, the aneurysm may continue to fill owing to collateral blood supply.
Giant aneurysms of the vertebrobasilar circulation are rare lesions without any optimal treatment. Despite advances with skull base approaches to these aneurysms, the surgical morbidity remains high. Coiling of giant aneurysms usually does not result in complete occlusion, and recanalization rates are high.23 The two goals for treatment of giant vertebrobasilar aneurysms are reduction of mass effect and persistent thrombosis of the lesion. Endovascular treatment of these challenging aneurysms is mostly limited to deconstructive approaches. For selected patients, unilateral or bilateral VA occlusion may be effective in excluding the aneurysm from the circulation or reducing the lesion size. Less commonly, the BA itself can be occluded proximal to the aneurysm, provided the patient has good collateral filling through the posterior communicating arteries (PCoAs). In general, the closer the parent vessel occlusion to the aneurysm, the higher the chances of complete aneurysm thrombosis. To this end, the endovascular approach may be more successful than surgical occlusion of the parent vessel. Endovascular trapping of the aneurysm is also possible in which the parent vessel is occluded proximal and distal to the aneurysm to prevent retrograde filling. Parent vessel occlusion that leads to partial thrombosis of giant aneurysms may be disastrous. Partially thrombosed giant aneurysms are susceptible to increased edema or SAH.24 Even for completely thrombosed aneurysms, it is important to recognize that clinical deterioration after parent vessel sacrifice is usually due to transient worsening of mass effect and not necessarily due to injury to perforators. However, these patients tend to improve, and endovascular parent artery occlusion leading to complete thrombosis of the aneurysm usually leads to good clinical outcomes.25
♦ Subsets of Posterior Fossa Aneurysms
Basilar Apex Aneurysms
The paradigm for the success of neuroendovascular therapy is the basilar apex aneurysm. Coil embolization undoubtedly has made the greatest impact on the treatment of this subset of intracranial aneurysms. Basilar apex aneurysms are, by far, the most common aneurysms of the posterior circulation.1 At many centers, including our own, these aneurysms are treated almost always by coiling. Our attitude is that endovascular options should be exhausted for this group of aneurysms before considering open surgical treatment. Stent and balloon-assisted coiling have allowed us to expand the indications for endovascular treatment to wide-necked and complex aneurysms. These advances, along with superior endovascular results, have made surgery for basilar apex aneurysms obsolete at many centers. Microcatheterization of these aneurysms is usually uneventful, as the BA usually has a straight course. The main challenge is preventing coil herniation and avoiding occlusion of the PCA.
Results from large endovascular series are favorable regarding technical success and occlusion rates for these aneurysms.18,26–28 Occlusion rates exceeding 90% can be achieved with primary coiling. In the largest series to date, complications occurred in 20% of 316 treated basilar apex aneurysms.26 Most of the complications were thromboembolic. Although many patients were lost to follow-up in this series, coil compaction and aneurysm recanalization was demonstrated in 24% at a mean follow-up of 19 months. Forty-eight patients (15%) required a second coiling session due to recanalization. These results may seem at first glance to be mediocre, but they include preliminary experience from the early 1990s.
Direct comparison between surgical and endovascular results for these aneurysms is difficult, as most published reports include selection bias toward a certain modality, based on aneurysm morphology and size. The reported morbidity in most surgical series for ruptured basilar apex aneurysms approaches 20%, with mortality approaching 10%.1,29,30 By comparison, endovascular morbidity and mortality is in the range of 10% and less than 5%, respectively.29,31,32 Stenting strategies usually involve placement of a single stent into the PCA that is at greater risk of occlusion. Stent delivery can be difficult if the PCA originates at a 90-degree angle from the BA. In rare cases, more creative configurations such as “Y-stenting” may be used if both PCAs are at risk of occlusion. In this strategy, the first stent is delivered into one PCA, and the second stent is delivered into the other PCA through the struts of the first stent.33 Another elegant yet potentially difficult technique is navigating a stent through the PCoA into the ipsilateral P1 segment and crossing the basilar apex into the contralateral P1 segment.34 The result is a stent that spans the neck of the aneurysm and protects both PCAs. Obviously, this strategy requires a relatively large, straight PCoA. If the stent placement is successful, a microcatheter can then be advanced via the vertebrobasilar route, through the horizontally oriented stent, and into the aneurysm for coiling. However, these extra vessel manipulations carry increased risk, and we favor the simplest strategies possible to accomplish aneurysm obliteration and parent vessel preservation.
Posterior Cerebral Artery Aneurysms
Aneurysms of the PCA represent approximately 1% of all intracranial aneurysms.3,35 These aneurysms are classified according to the segment of the PCA involved, that is, P1, P1– PCoA junction, P2A, P2B, P3, or P4. Most PCA aneurysms arise from the P1 and P2 segments. PCA aneurysms tend to occur in younger patients, have a higher incidence of being large or giant than aneurysms at other locations, and often occur in the setting of other cerebrovascular lesions.36,37 Aneurysms of the P2 segment and beyond do not carry the same surgical prognosis as do those of the P1 segment.37 The P2 segment, which extends from the PCoA–PCA junction to the origin of the inferior temporal arteries in the quadrigeminal cistern, may give rise to aneurysms less frequently. Most reports of peripheral PCA aneurysms are comprised of small series or single cases (Fig. 21.4). P2 aneurysms are usually saccular, but fusiform and dissecting aneurysms are also common in this location.36,37 Compared with surgery, endovascular treatment of P2 aneurysms offers several advantages. The P2 segment is roughly 3 mm in diameter, making endovascular navigation possible.38 Because aneurysms of the P2 segment tend to be wide-necked, serpentine, or fusiform, endovascular preservation of the parent vessel is usually not possible. Balloon test occlusion of the P2 segment does not seem to be reliable. Instead, evaluation of the collateral network between the PCA and other vascular territories is more predictive of the safety of P2 sacrifice. On the basis of small series, it appears that sacrifice of the P2 segment, including the P2A subsegment, is safe and does not result in visual field deficits.37 In fact, occlusion appears to be safer in the proximal P2A segment because of preserved collateral supply distally from the anterior choroidal artery, splenial branches of the anterior cerebral artery, and leptomeningeal collaterals from the middle cerebral artery.37
Posterior Inferior Cerebellar Artery Aneurysms
Aneurysms of the PICA account for roughly 0.5% of all intracranial aneurysms.39 The morbidity associated with open surgery for these lesions has been reported to be as high as 66%, with most complications due to lower cranial nerve injuries.2,4 Although the vast majority of patients with unruptured PICA aneurysms or good-grade SAH ultimately recover well after surgery, endovascular therapy of these aneurysms has proven to reduce the treatment-associated morbidity, and long-term results are comparable to those with clipping.40 Technical success with coiling of these aneurysms typically exceeds 95% in our experience and that of others (Fig. 21.5). Procedure-related morbidity, in the form of thromboembolism or intraprocedural aneurysm rupture, ranges from 10 to 15%.40,41 Endovascular results for ruptured PICA aneurysms are especially favorable, as almost 87% of patients with Hunt-Hess grades I, II, or III have good long-term clinical outcomes. In contrast to surgical results, half of the patients with poor Hunt-Hess grades had good clinical outcomes in one recent report.41 At our institution, the proximal PICA aneurysm is the main posterior circulation aneurysm that is still occasionally treated with surgery, but even this practice pattern is shifting toward endovascular therapy, considering these good angiographic and clinical results.
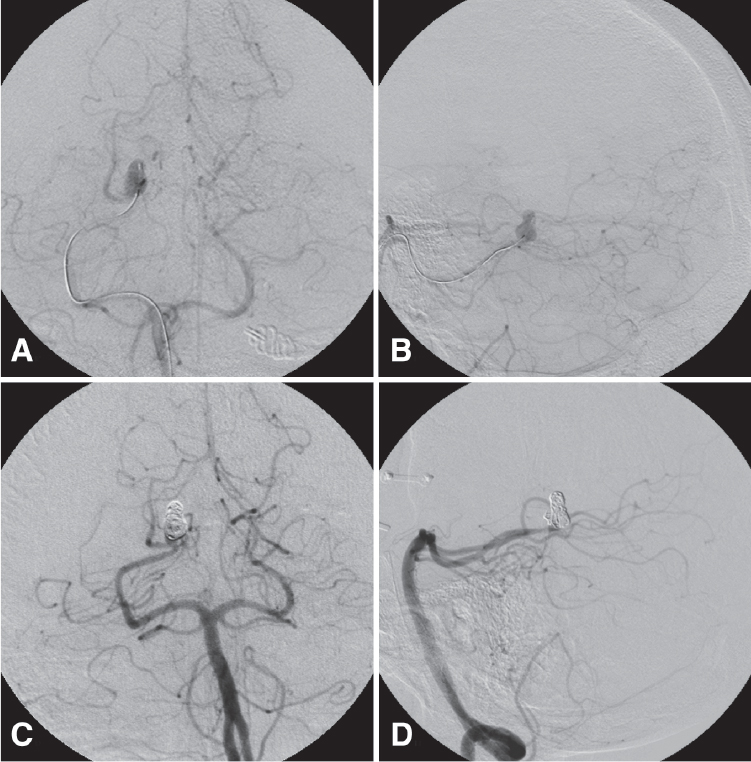
Fig. 21.4 Workup of chronic headaches in this 60-year-old woman led to the discovery of an unruptured right PCA aneurysm arising from the P3 segment. The aneurysm appeared irregular but had a discrete neck and was treated with direct coil embolization. Projections of the right VA injection (A, AP; and B, lateral) show microcatheterization of the aneurysm. Views (C, AP; and D, lateral) after completion of coiling demonstrate a small neck remnant and preservation of the distal PCA.
Most PICA aneurysms occur proximal to the choroidal point. The most important technical pearl regarding coiling of these proximal PICA aneurysms is preservation of the parent vessel because of brainstem perforators that arise from the anterior and lateral medullary segments of the parent vessel. Distal to the choroidal point, the PICA can usually be sacrificed with impunity.42 Endovascular treatment of unruptured proximal PICA aneurysms with wide necks usually requires stent assistance or partial coiling in the acute SAH setting, followed by stent assistance in a delayed fashion.
Peripheral Aneurysms of the Posterior Circulation
Aneurysms of the peripheral cerebellar arteries represent less than 1% of all aneurysms, with the majority located on the PICA.35 Most peripheral aneurysms in the posterior circulation tend to be traumatic or mycotic. Direct surgical or endovascular treatment of these lesions with preservation of the parent artery is possible but can be very difficult. These aneurysms can often be treated safely and effectively with proximal occlusion of the parent vessel or trapping. These indirect techniques may be necessary in treating mycotic or traumatic aneurysms and saccular aneurysms with wide necks. An endovascular approach is attractive and appears to be as effective as surgical sacrifice of the parent artery. Test occlusion is often not technically feasible or practical, as it does not appear to be a reliable predictor of expected deficits from parent artery sacrifice in the peripheral cerebellar arteries because collateral circulation is usually adequate.43
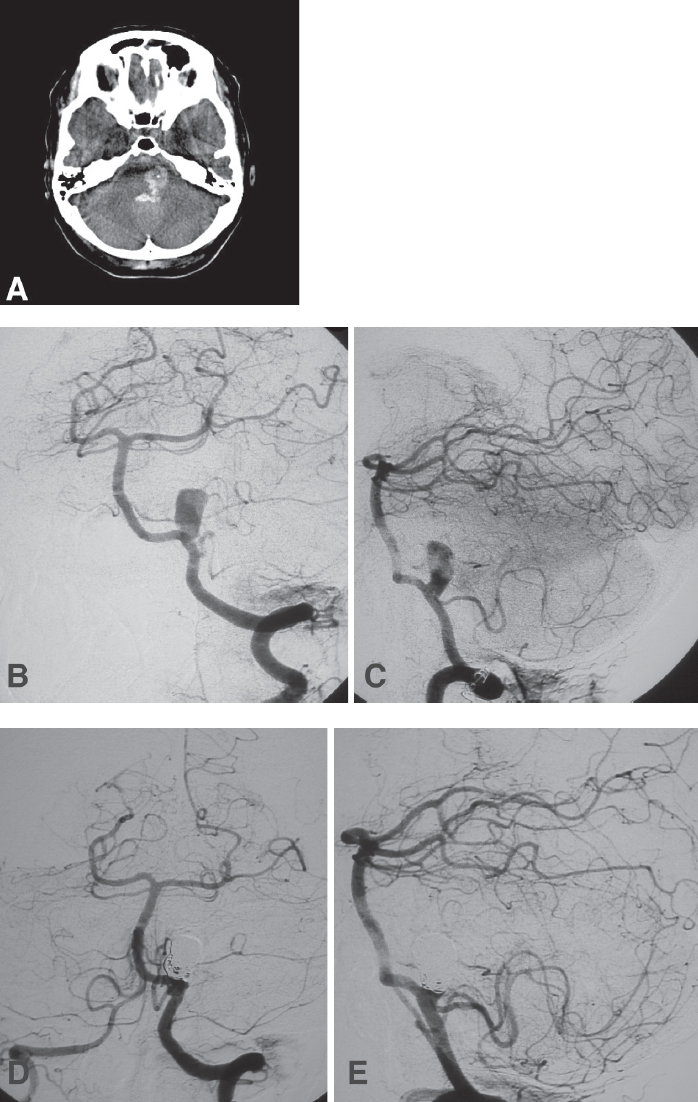
Fig. 21.5 A 50-year-old woman presented with grade V SAH and transiently improved after ventriculostomy. (A) Noncontrast computed tomography (CT) scan of the head shows hemorrhages in the fourth ventricle and lateral medullary cistern. (B,C) Angiography confirmed the presence of a small-necked aneurysm at the junction of the left VA and the posterior inferior cerebellar artery (PICA). (D,E) Because of the patient’s poor clinical status, the aneurysm was coiled directly, leaving a small residual neck. The patient ultimately died within 1 week due to intractable intracranial hypertension.
Peripheral aneurysms of the SCA are extremely rare. Patients with these aneurysms usually present with SAH or fourth cranial nerve palsy.44 Occlusion of the SCA seems to be well tolerated because of adequate collateral supply to the distal territory of this artery. Furthermore, there are few perforators to the brainstem originating from the peripheral segments of the SCA.45,46 The endovascular approach to these lesions involves selective microcatheterization of the distal SCA. Although direct treatment of these aneurysms with coils is optimal, this is usually not possible because of their lack of discrete necks. Endovascular occlusion of the parent artery should be done as close to the aneurysm as possible to minimize the length of arterial occlusion and to reduce the risk of occlusion of uninvolved branches.
Aneurysms of the distal AICA are equally rare with fewer than 100 reported cases.43 The AICA is the least likely of the major posterior circulation arteries to harbor an aneurysm. In addition to producing SAH, these lesions may produce symptoms such as tinnitus, vertigo, facial weakness, and hearing loss due to their location in the cerebellopontine angle.47 Endovascular treatment of these aneurysms involves either direct coil embolization of the lesion or occlusion of the distal AICA.47,48 A recurrent theme in treating peripheral aneurysms of the posterior fossa is that parent artery occlusion is safe as long as the aneurysm is distal to brainstem perforators. As with all endovascular arterial occlusions, there is a theoretical risk of retrograde thrombosis.
♦ Fusiform and Dissecting Aneurysms
Dissecting aneurysms of the posterior circulation are also uncommon, but they accounted for 4.5% of nontraumatic SAH in one autopsy series. However, the true incidence is not known and likely much lower than this figure.49 Due to its thin media and adventitia, the intradural VA is more susceptible to dissections than its extradural counterpart.50 Recurrent hemorrhages from dissecting VA aneurysms range from 30 to 70%. Mortality from untreated lesions has been reported to be 50%.50 Lesions with the highest risk involve the dominant VA with inadequate collateral supply from the contralateral VA or the PCoAs. Originally, endovascular treatment for these lesions was limited to deconstructive techniques (see above). These techniques include proximal occlusion of the affected segment of artery by coil embolization or endovascular trapping of the diseased segment. As with giant aneurysms, surgical bypass adjuncts may be necessary. One series of these deconstructive techniques yielded good or excellent clinical results in 61% and angiographic cure in 79% of patients.50
Endovascular parent artery occlusion was evaluated in a series of 13 patients with dissecting or fusiform vertebrobasilar aneurysms.51 The clinical results were favorable for aneurysms in which complete thrombosis was achieved. Aneurysms involving only one VA were in this group. However, mortality exceeded 50% in the group in which only partial thrombosis occurred because of involvement of the BA or both VAs.51 The most secure deconstructive treatment may be aneurysm trapping, as proximal occlusion alone may result in recanalization from retrograde filling.52
Reconstructive techniques with stenting could be an attractive alternative for treating these difficult lesions. Stenting of these lesions with or without coiling may cause altered hemodynamics, resulting in aneurysm thrombosis and preservation of the parent vessel. Essentially, the stent reconstructs the original parent vessel while the aneurysm outside of the stent becomes thrombosed. The addition of coils may promote aneurysm thrombosis (Fig. 21.6). Preliminary experience indicates a high technical success rate and good short-term results.53,54
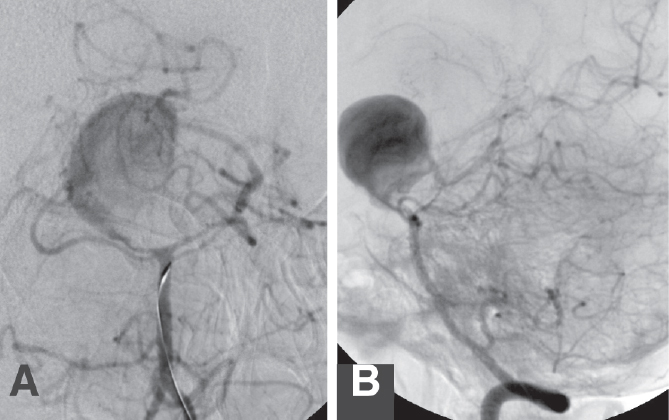
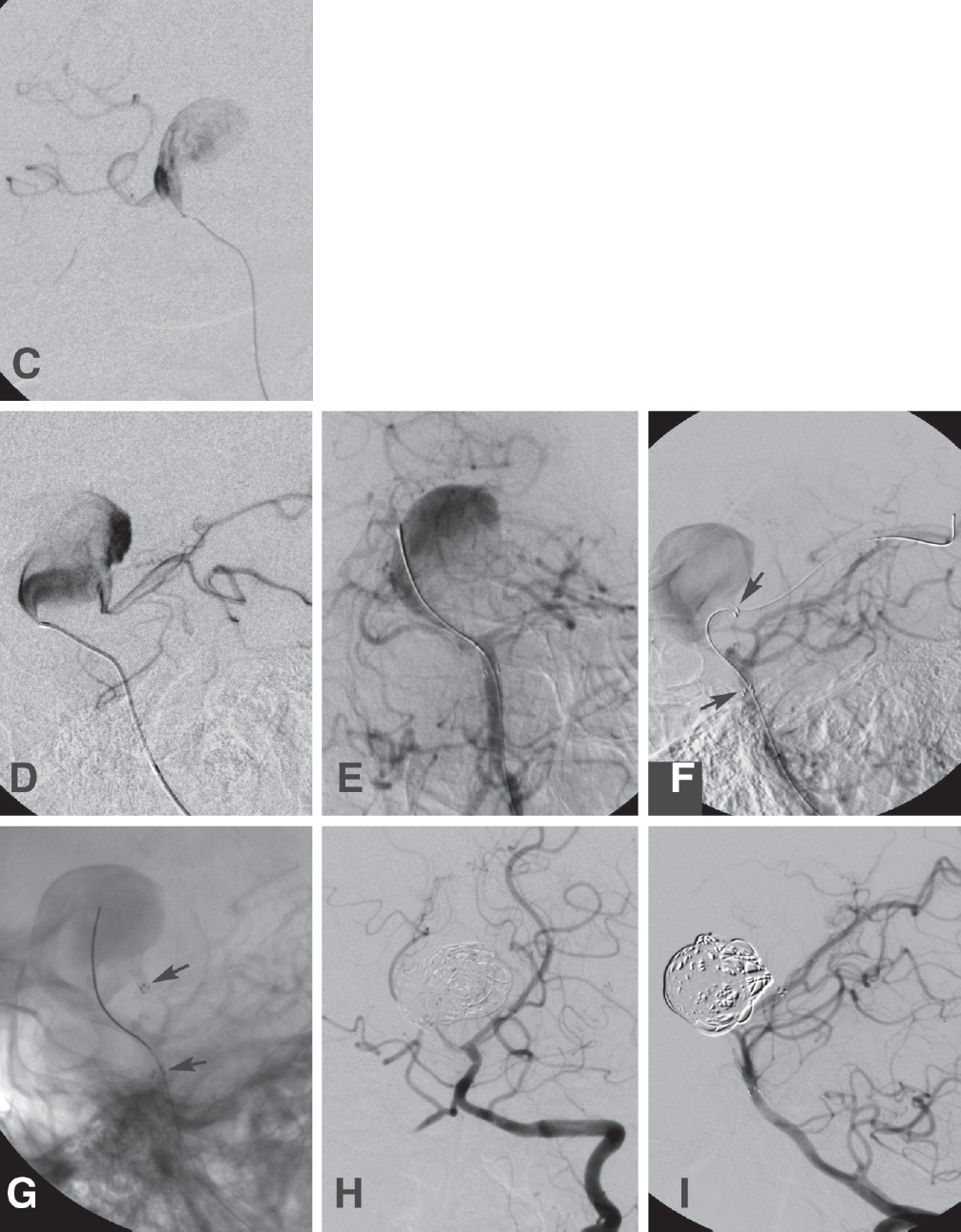
Fig. 21.6 A 43-year-old woman presented with dull headaches for 2 weeks. There was no evidence of SAH on noninvasive studies. Angiography of a left VA injection (A, AP; and B, lateral), confirmed the presence of a giant, seemingly fusiform left PCA aneurysm. The right PCA did not fill from the anterior circulation. There was vasospasm of the PCAs. Microcatheterization of the aneurysm (C, AP; and D, lateral) confirmed its fusiform morphology. Endovascular treatment was done in two stages. (E,F) First, a Neuroform stent (Boston Scientific) was placed through the fusiform aneurysm. (F) The arrows point to the stent markers). (G) The patient returned later for coil embolization through the stent. This is the unsubtracted view; the arrows point to the stent markers. One week later, the patient developed right hemiparesis from vasospasm of the left middle cerebral artery, suggesting that the aneurysm had ruptured previously. (H,I) Four months later, the patient had a very mild right hemiparesis; angiography showed a small, stable aneurysm neck remnant.
♦ Conclusion
Endovascular treatment of posterior circulation aneurysms has supplanted surgical therapy as a first choice in many centers. Although certain aneurysms are not amenable to direct coil embolization, the expansion of endovascular techniques has allowed for treatment of broad-necked lesions. Although deconstructive approaches are appropriate for some fusiform and dissecting aneurysms, endovascular stent reconstruction seems promising for these difficult lesions. Increased aneurysm recurrence rates and thromboembolic complications are the main drawbacks to endovascular treatment, and these considerations must be weighed against the morbidity of surgical treatment.
References
1. Yasargil M. Microneurosurgery. New York: Thieme, 1984
2. Al-khayat H, Al-Khayat H, Beshay J, Manner D, White J. Vertebral arteryposteroinferior cerebellar artery aneurysms: clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery 2005;56:2–10, discussion 11 PubMed
3. Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg 1979;26:96–144 PubMed
4. Horowitz M, Kopitnik T, Landreneau F, et al. Posteroinferior cerebellar artery aneurysms: surgical results for 38 patients. Neurosurgery 1998; 43:1026–1032 PubMed
5. Molyneux A, Kerr R, Stratton I, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–1274 PubMed
6. Molyneux AJ, Kerr RS, Yu LM, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–817 PubMed
7. Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 1998;43:1281–1295, discussion 1296–1297 PubMed
8. Cloft HJ, Joseph GJ, Tong FC, Goldstein JH, Dion JE. Use of three-dimensional Guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. AJNR Am J Neuroradiol 2000;21:1312–1314 PubMed
9. Vallée JN, Pierot L, Bonafé A, et al. Endovascular treatment of intracranial wide-necked aneurysms using three-dimensional coils: predictors of immediate anatomic and clinical results. AJNR Am J Neuroradiol 2004;25:298–306 PubMed
10. Moret J, Cognard C, Weill A, Castaings L, Rey A. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol 1997;24:30–44 PubMed
11. Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Balloon-assisted coil embolization of intracranial aneurysms: incidence, complications, and angiography results. J Neurosurg 2006;105:396–399 PubMed
12. Benitez RP, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery 2004;54: 1359–1367, discussion 1368 PubMed
13. Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng 1997;25:460–469 PubMed
14. Zenteno MA, Murillo-Bonilla LM, Guinto G, et al. Sole stenting bypass for the treatment of vertebral artery aneurysms: technical case report. Neurosurgery 2005;57(1, Suppl)E208, discussion E208 PubMed
15. Akpek S, Arat A, Morsi H, Klucznick RP, Strother CM, Mawad ME. Self-expandable stent-assisted coiling of wide-necked intracranial aneurysms: a single-center experience. AJNR Am J Neuroradiol 2005;26: 1223–1231 PubMed
16. Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3-6-mo) follow-up. Neurosurgery 2005;56:1191–1201, discussion 1201–1202 PubMed
17. Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg 2002;97:1306–1313 PubMed
18. Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 PubMed
19. Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997;41:1235–1245, discussion 1245–1246 PubMed
20. Fox AJ, Viñuela F, Pelz DM, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg 1987;66:40–46 PubMed
21. Higashida RT, Halbach VV, Dowd CF, Barnwell SL, Hieshima GB. Intracranial aneurysms: interventional neurovascular treatment with detachable balloons—results in 215 cases. Radiology 1991;178:663–670 PubMed
22. Aymard A, Gobin YP, Hodes JE, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg 1991;74:393–398 PubMed
23. Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803, discussion 803–804 PubMed
24. Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg 1993;79: 161–173 PubMed
25. Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. Giant vertebrobasilar aneurysms: endovascular treatment and long-term follow-up. Neurosurgery 2004;55:316–323, discussion 323–326 PubMed
26. Henkes H, Fischer S, Mariushi W, et al. Angiographic and clinical results in 316 coil-treated basilar artery bifurcation aneurysms. J Neurosurg 2005;103:990–999 PubMed
27. Mordasini P, Schroth G, Guzman R, Barth A, Seiler RW, Remonda L. Endovascular treatment of posterior circulation cerebral aneurysms by using Guglielmi detachable coils: a 10-year single-center experience with special regard to technical development. AJNR Am J Neuroradiol 2005;26:1732–1738 PubMed
28. Pandey AS, Koebbe C, Rosenwasser RH, Veznedaroglu E. Endovascular coil embolization of ruptured and unruptured posterior circulation aneurysms: review of a 10-year experience. Neurosurgery 2007;60:626– 636, discussion 636–637 PubMed
29. Gruber DP, Zimmerman GA, Tomsick TA, van Loveren HR, Link MJ, Tew JM Jr. A comparison between endovascular and surgical management of basilar artery apex aneurysms. J Neurosurg 1999;90:868–874 PubMed
30. Peerless SJ, Wallace MD, Drake CG. Giant intracranial aneurysms. In: Youmans JR, ed. Neurological Surgery, 3rd ed. Philadelphia: WB Saunders, 1990:1742–1763
31. McDougall CG, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393–399 PubMed
32. Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–1403 PubMed
33. Perez-Arjona E, Fessler RD. Basilar artery to bilateral posterior cerebral artery ‘Y stenting’ for endovascular reconstruction of wide-necked basilar apex aneurysms: report of three cases. Neurol Res 2004;26:276– 281 PubMed
34. Wanke I, Gizewski E, Forsting M. Horizontal stent placement plus coiling in a broad-based basilar-tip aneurysm: an alternative to the Y-stent technique. Neuroradiology 2006;48:817–820 PubMed
35. Drake CG, Amacher AL. Aneurysms of the posterior cerebral artery. J Neurosurg 1969;30:468–474 PubMed
36. Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR Am J Neuroradiol 2001;22:27–34 PubMed
37. Hallacq P, Piotin M, Moret J. Endovascular occlusion of the posterior cerebral artery for the treatment of p2 segment aneurysms: retrospective review of a 10-year series. AJNR Am J Neuroradiol 2002;23:1128– 1136 PubMed
38. Zeal AA, Rhoton AL Jr. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg 1978;48:534–559 PubMed
39. Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg 1966;25:219–239 PubMed
40. Mukonoweshuro W, Laitt RD, Hughes DG. Endovascular treatment of PICA aneurysms. Neuroradiology 2003;45:188–192 PubMed
41. Mericle RA, Reig AS, Burry MV, Eskioglu E, Firment CS, Santra S. Endovascular surgery for proximal posterior inferior cerebellar artery aneurysms: an analysis of Glasgow Outcome Score by Hunt-Hess grades. Neurosurgery 2006;58:619–625, discussion 619–625 PubMed
42. Hudgins RJ, Day AL, Quisling RG, Rhoton AL Jr, Sypert GW, Garcia-Bengochea F. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg 1983;58:381–387 PubMed
43. Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. Endovascular treatment of peripheral cerebellar artery aneurysms. AJNR Am J Neuroradiol 2003;24:1208–1213 PubMed
44. Collins TE, Mehalic TF, White TK, Pezzuti RT. Trochlear nerve palsy as the sole initial sign of an aneurysm of the superior cerebellar artery. Neurosurgery 1992;30:258–261 PubMed
45. Chaloupka JC, Putman CM, Awad IA. Endovascular therapeutic approach to peripheral aneurysms of the superior cerebellar artery. AJNR Am J Neuroradiol 1996;17:1338–1342 PubMed
46. Gács G, Viñuela F, Fox AJ, Drake CG. Peripheral aneurysms of the cerebellar arteries. Review of 16 cases. J Neurosurg 1983;58:63–68 PubMed
47. Zager EL, Shaver EG, Hurst RW, Flamm ES. Distal anterior inferior cerebellar artery aneurysms. Report of four cases. J Neurosurg 2002;97:692– 696 PubMed
48. Suzuki K, Meguro K, Wada M, Fujita K, Nose T. Embolization of a ruptured aneurysm of the distal anterior inferior cerebellar artery: case report and review of the literature. Surg Neurol 1999;51:509–512 PubMed
49. Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 1991;75:874–882 PubMed
50. Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 2003;24:1421–1428 PubMed
51. Leibowitz R, Do HM, Marcellus ML, Chang SD, Steinberg GK, Marks MP. Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. AJNR Am J Neuroradiol 2003;24:902–907 PubMed
52. Chang SD, Marks MP, Steinberg GK. Recanalization and rupture of a giant vertebral artery aneurysm after hunterian ligation: case report. Neurosurgery 1999;44:1117–1120, discussion 1120–1121 PubMed
53. Chiaradio JC, Guzman L, Padilla L, Chiaradio MP. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery 2002; 50:213–216, discussion 216–217 PubMed
54. Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg 2001;94:427–432 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








